This impurity is related to N-Nitroso Tinidazole Impurity A is provided with comprehensive characterization data in accordance with regulatory guidelines. It is suitable for use in analytical method development, method validation (AMV), Quality Control (QC) applications for Abbreviated New Drug Applications (ANDA) and commercial production of Asenapine.
N-Nitroso Tinidazole Impurity A is used as a reference standard in analytical research. It ensures consistency of formulations.
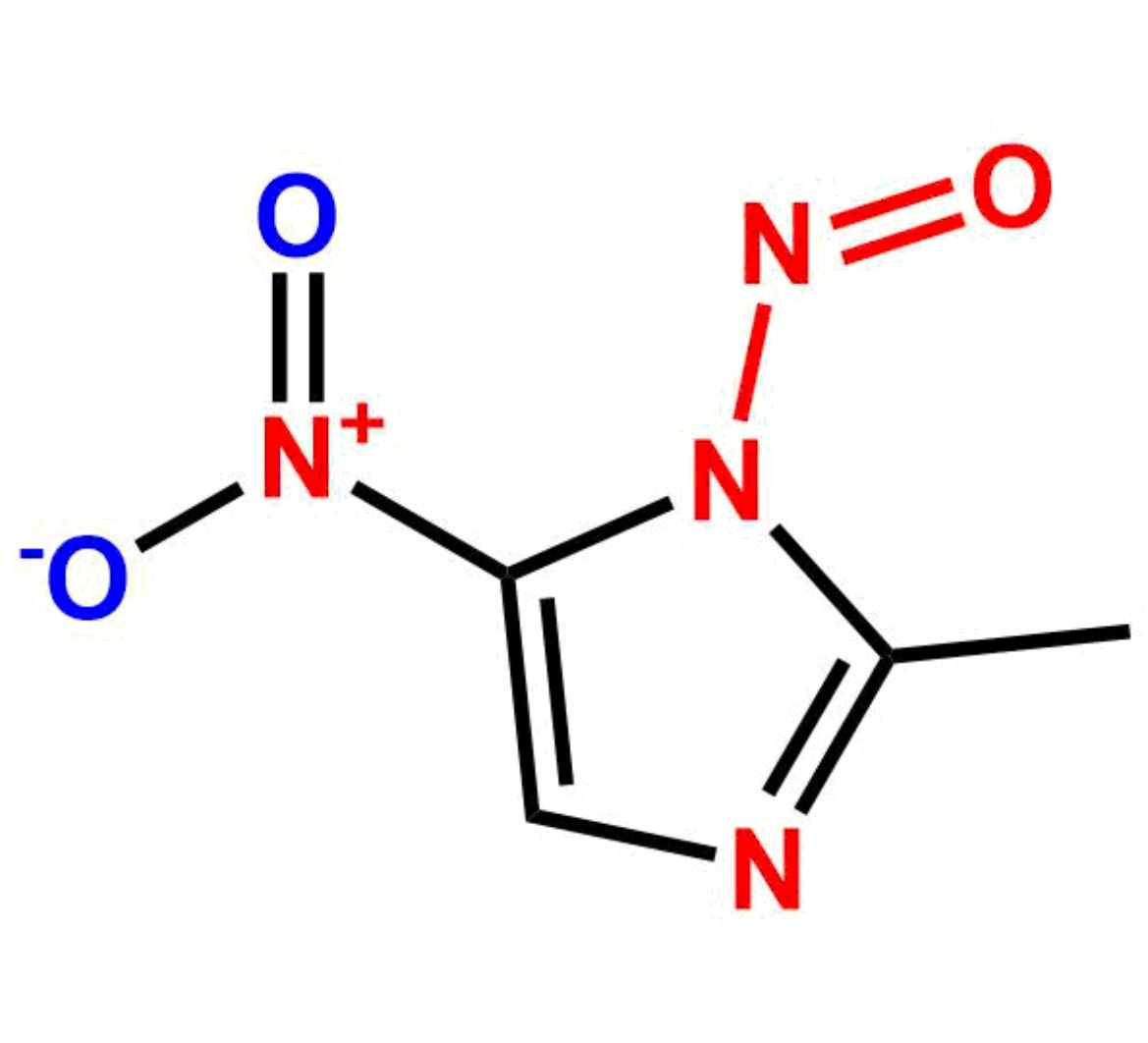
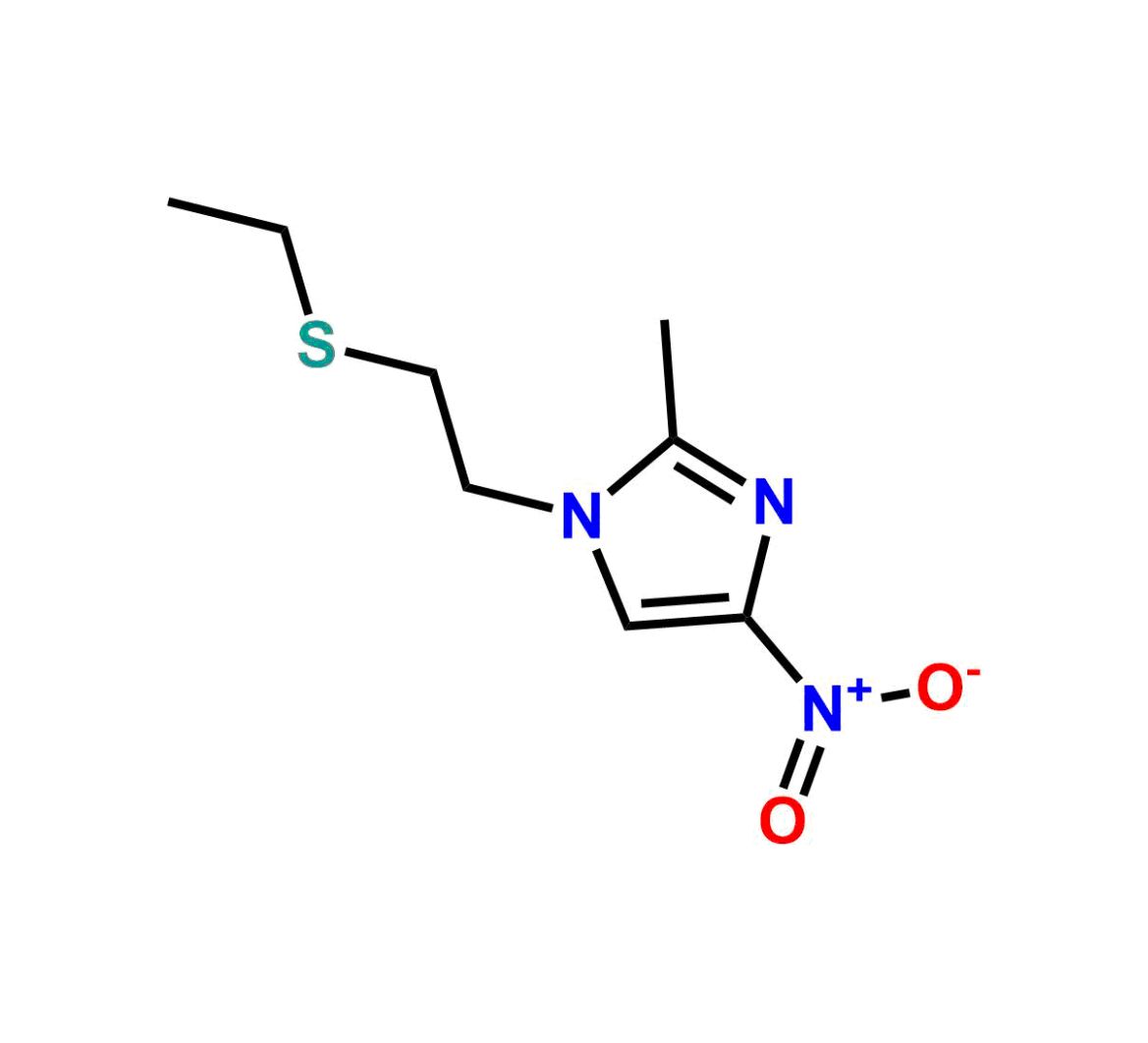
Chemical Name:2-methyl-5-nitro-1-nitroso-1H-imidazole Country of Origin: India Product Category: Impurity Reference StandardAPI NAME: Tinidazole Molecular Formula: C4H4N4O3
Molecular Weight: 156.1
Storage: Store in a cool, dry place.