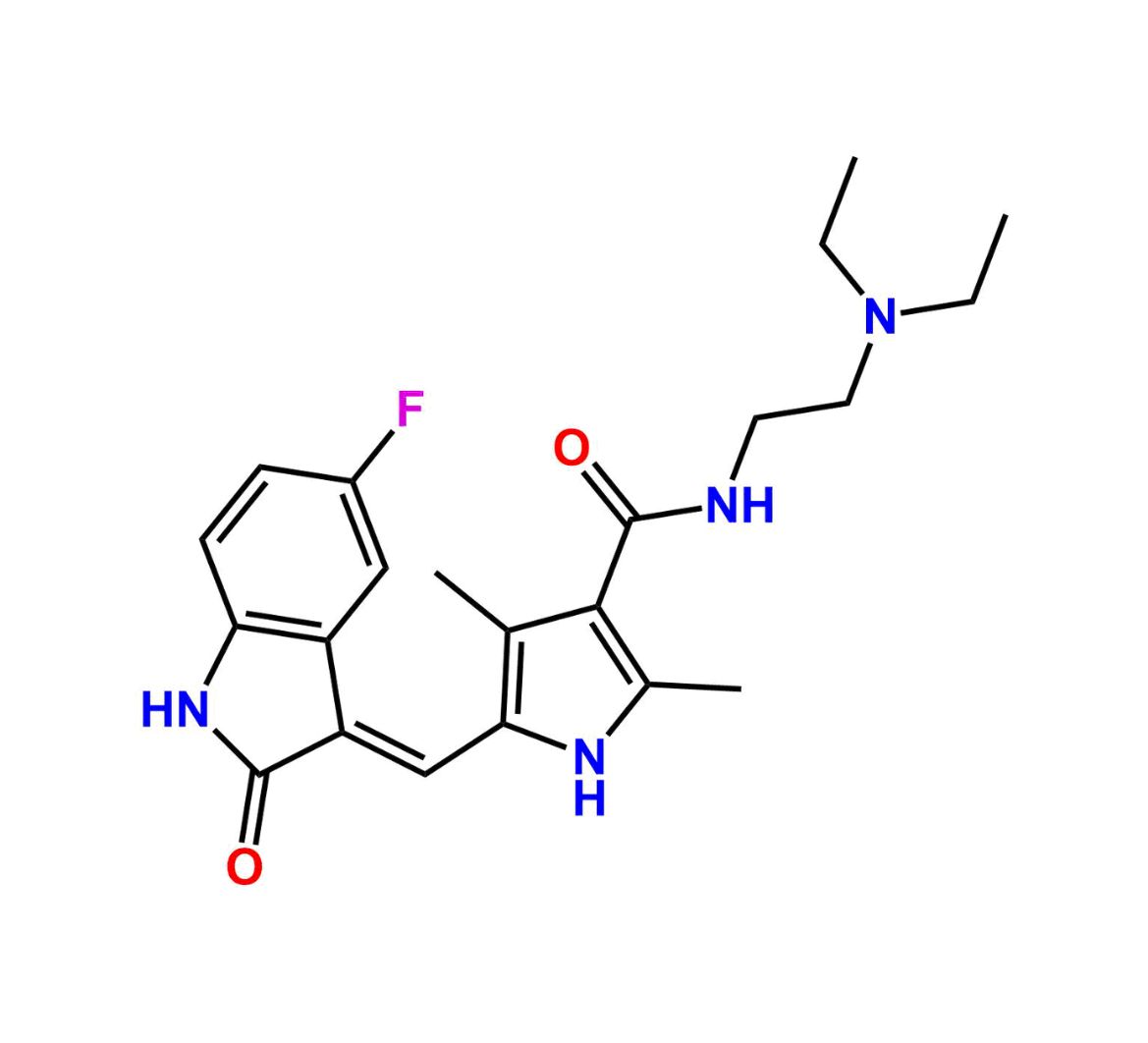
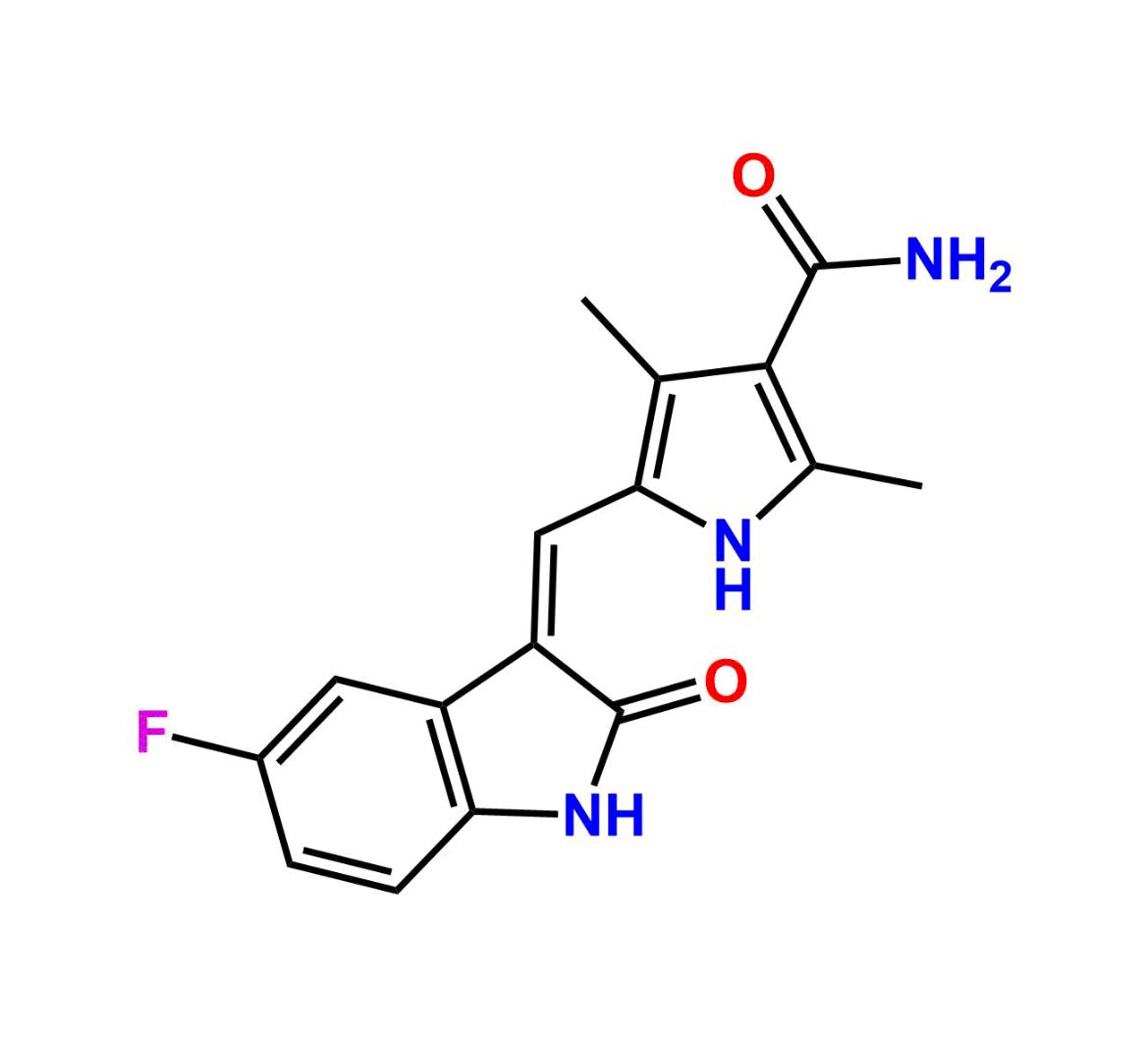
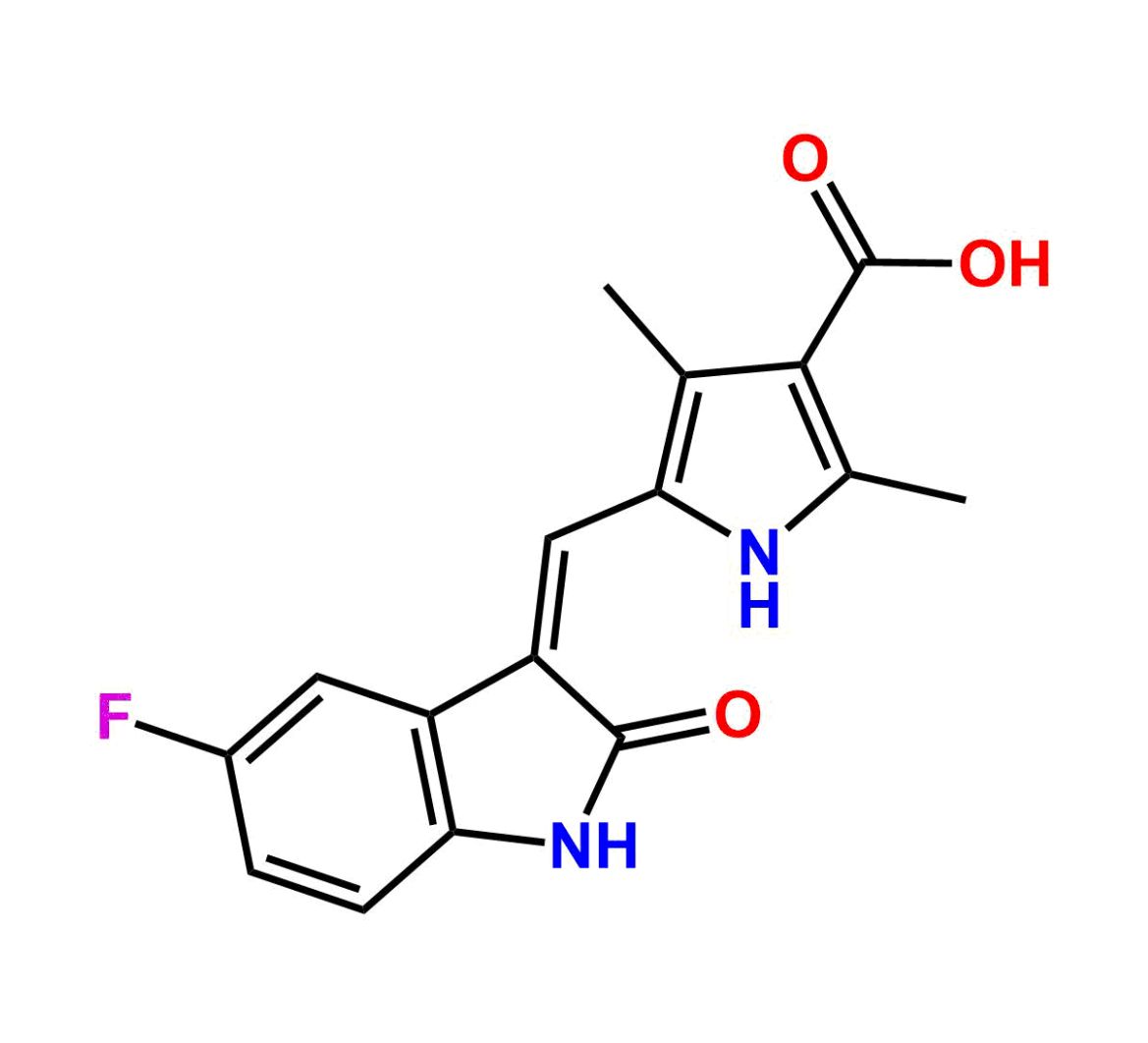
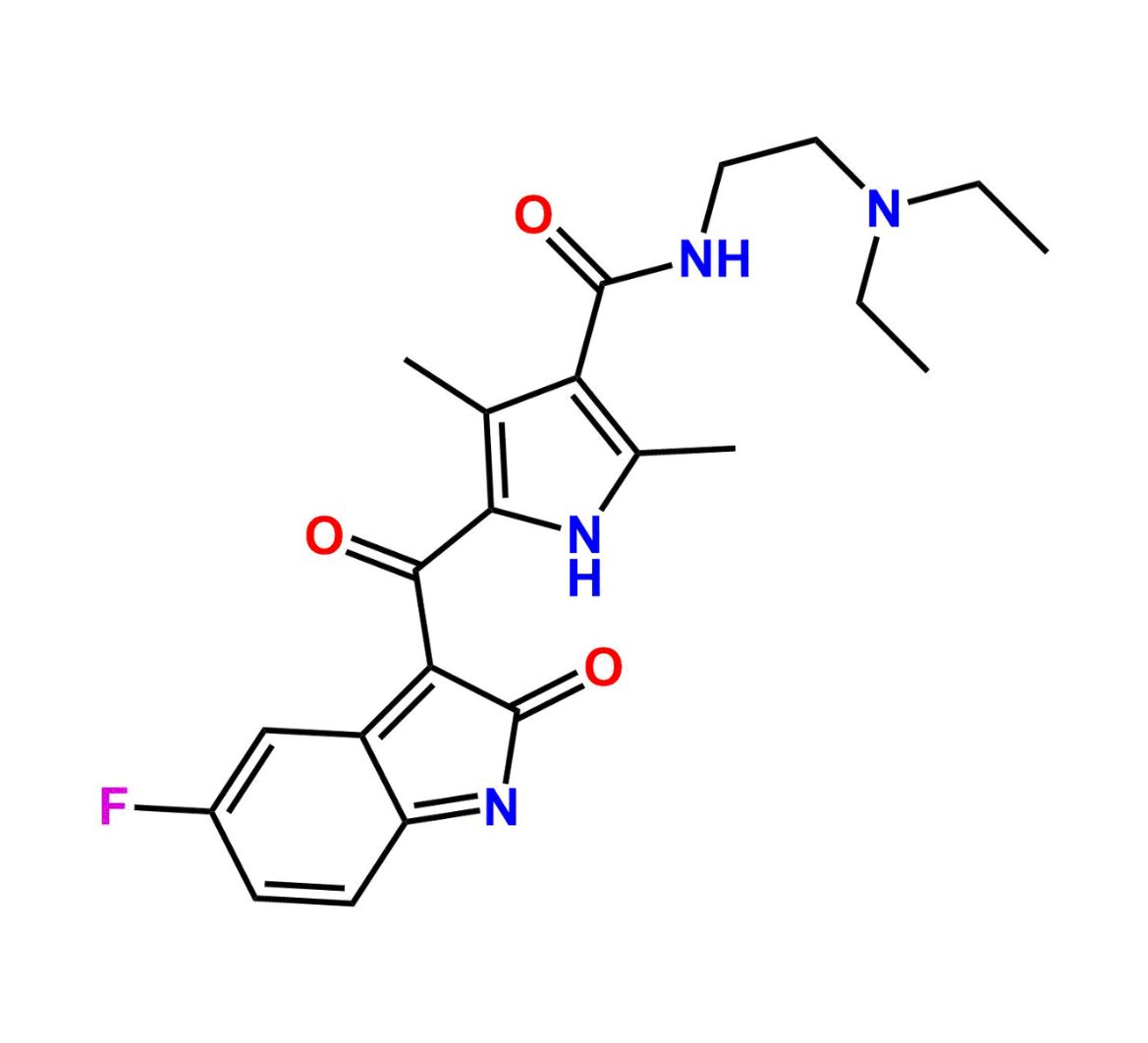
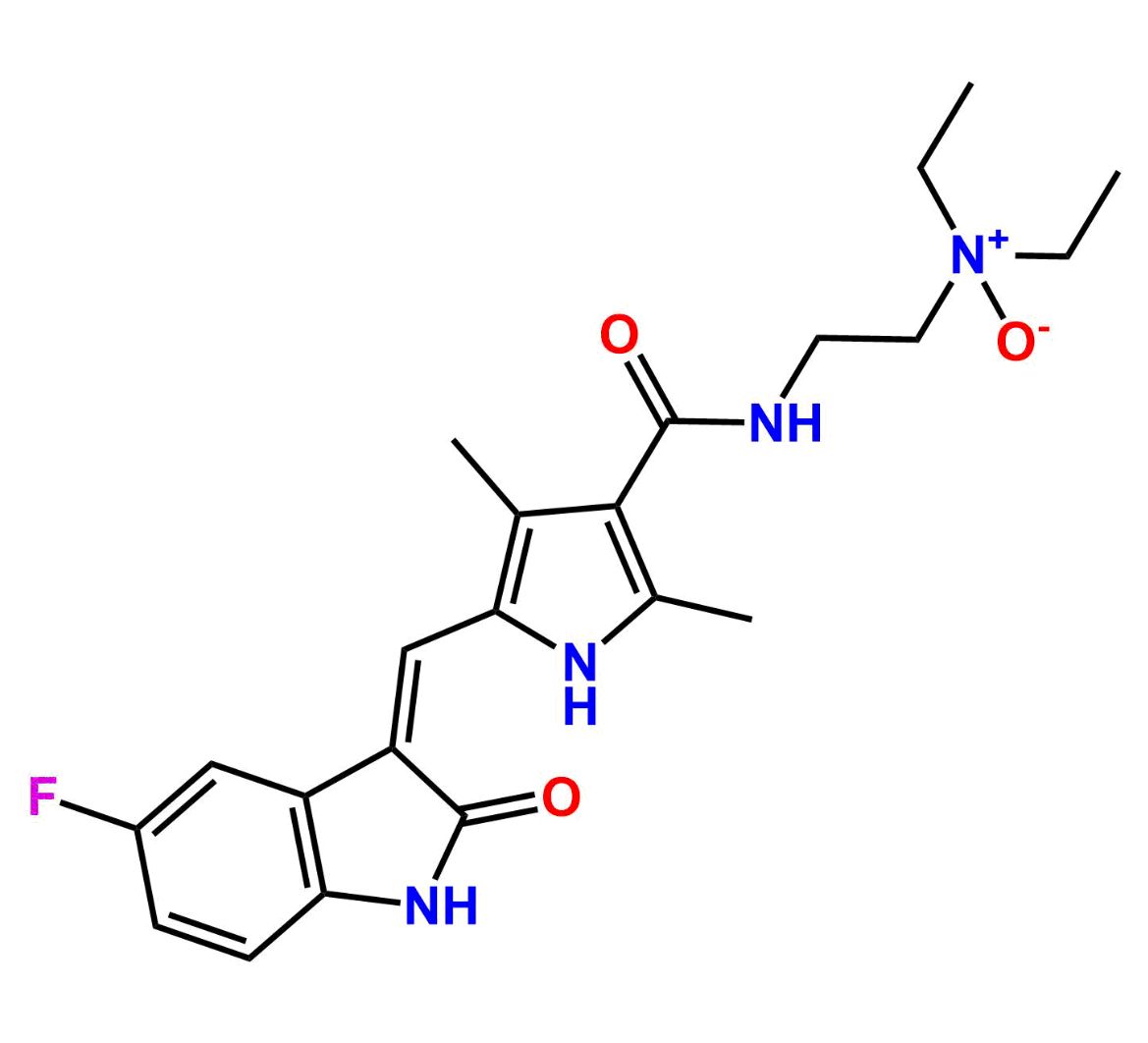
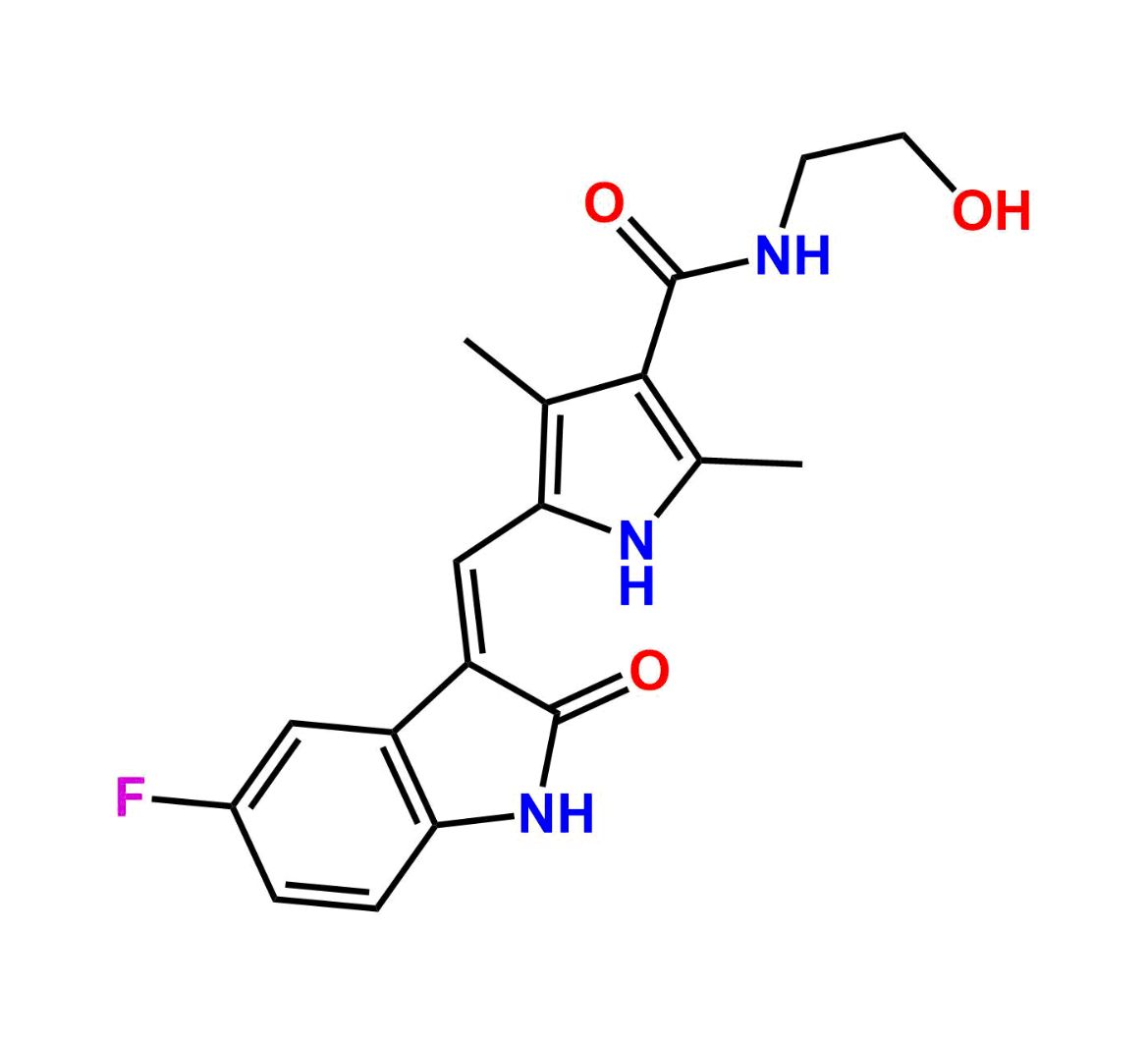
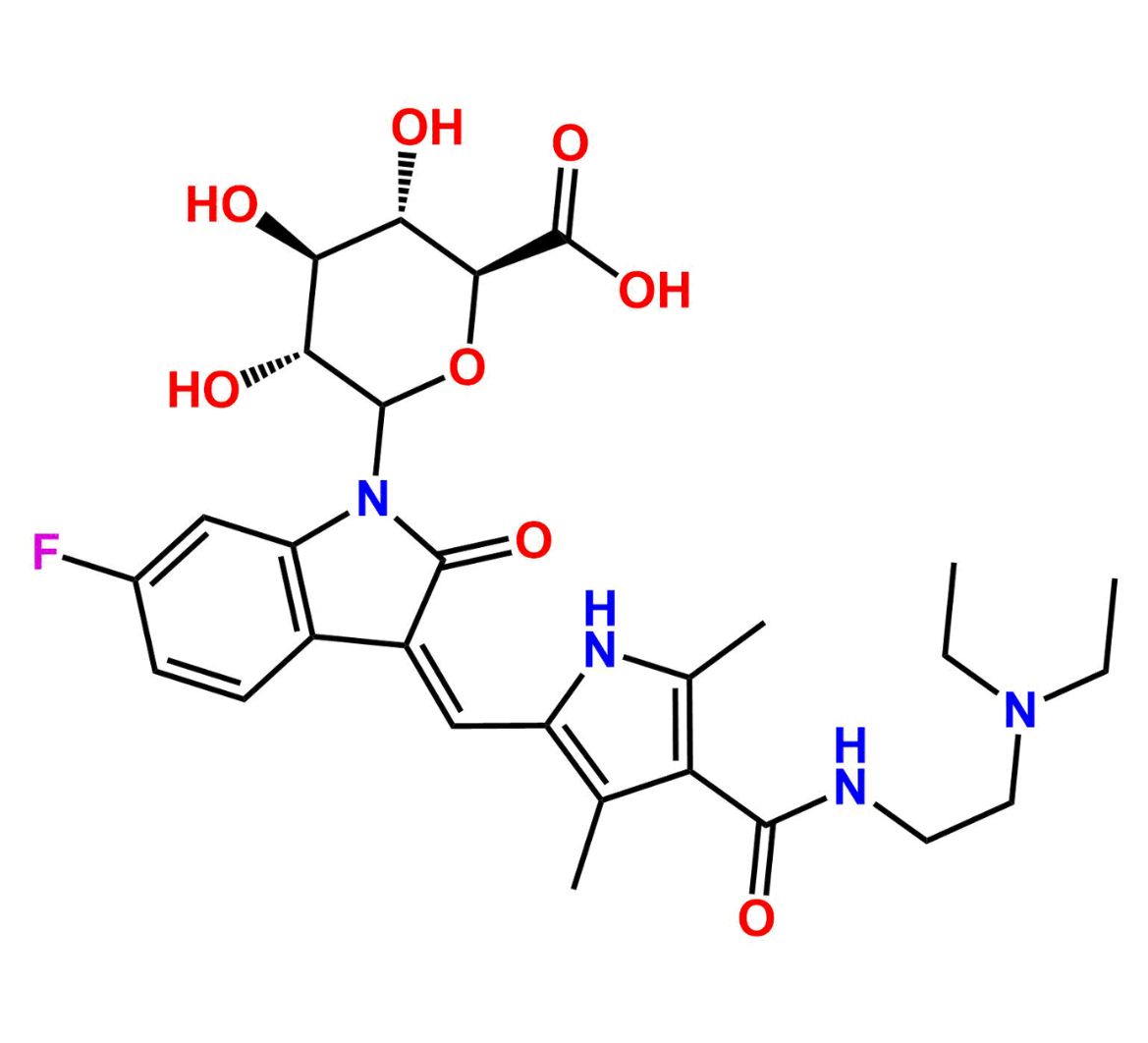
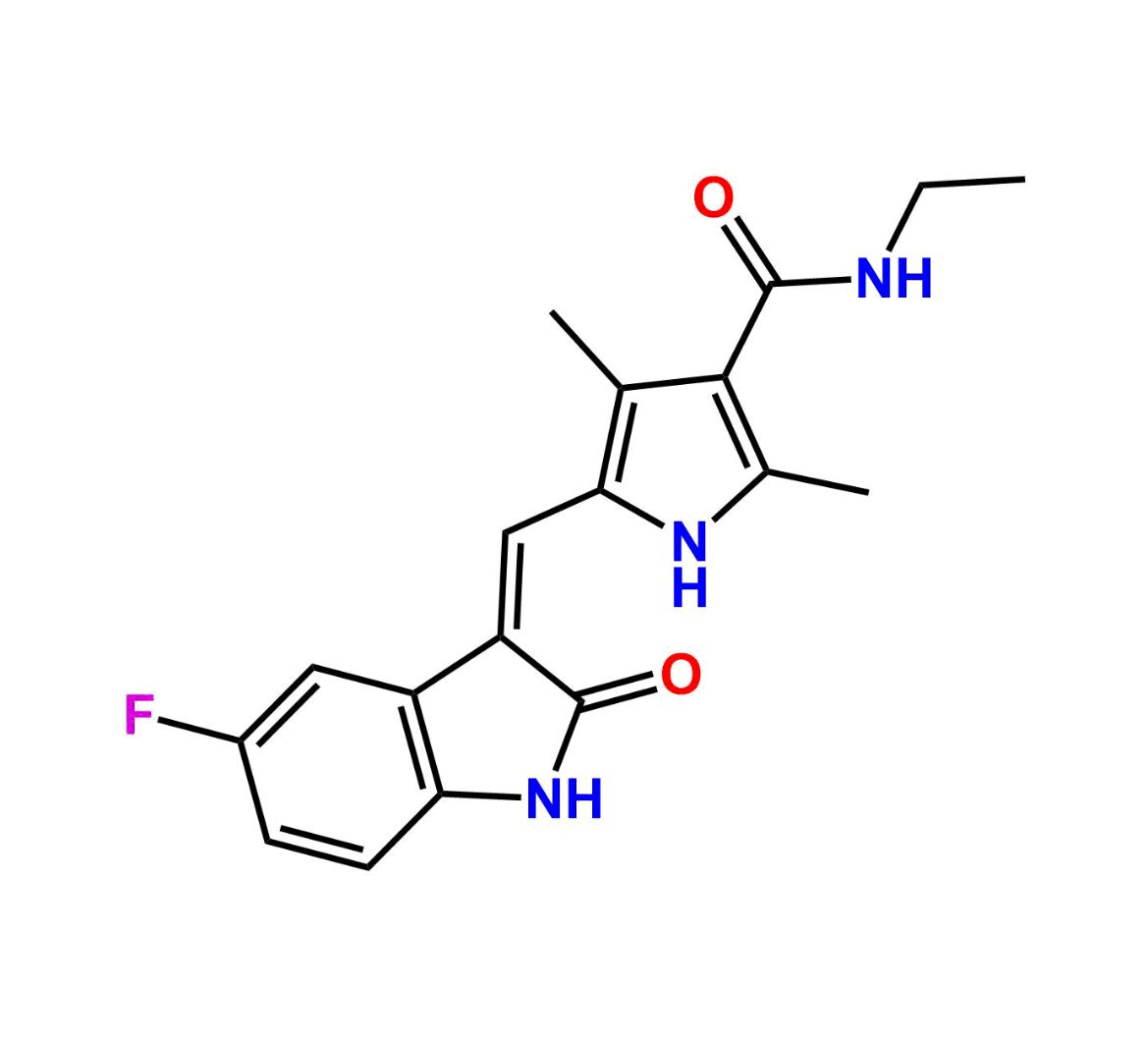
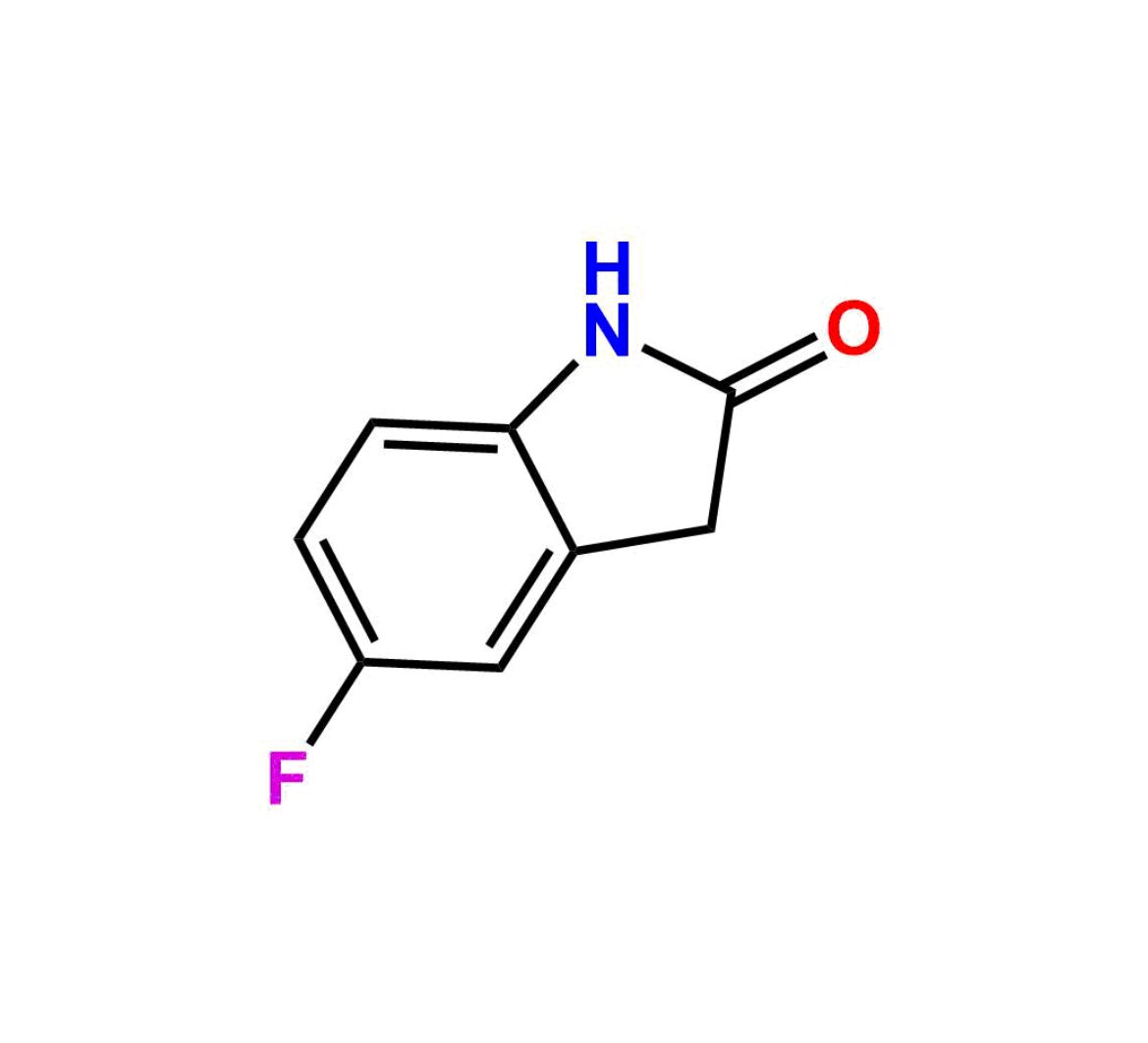
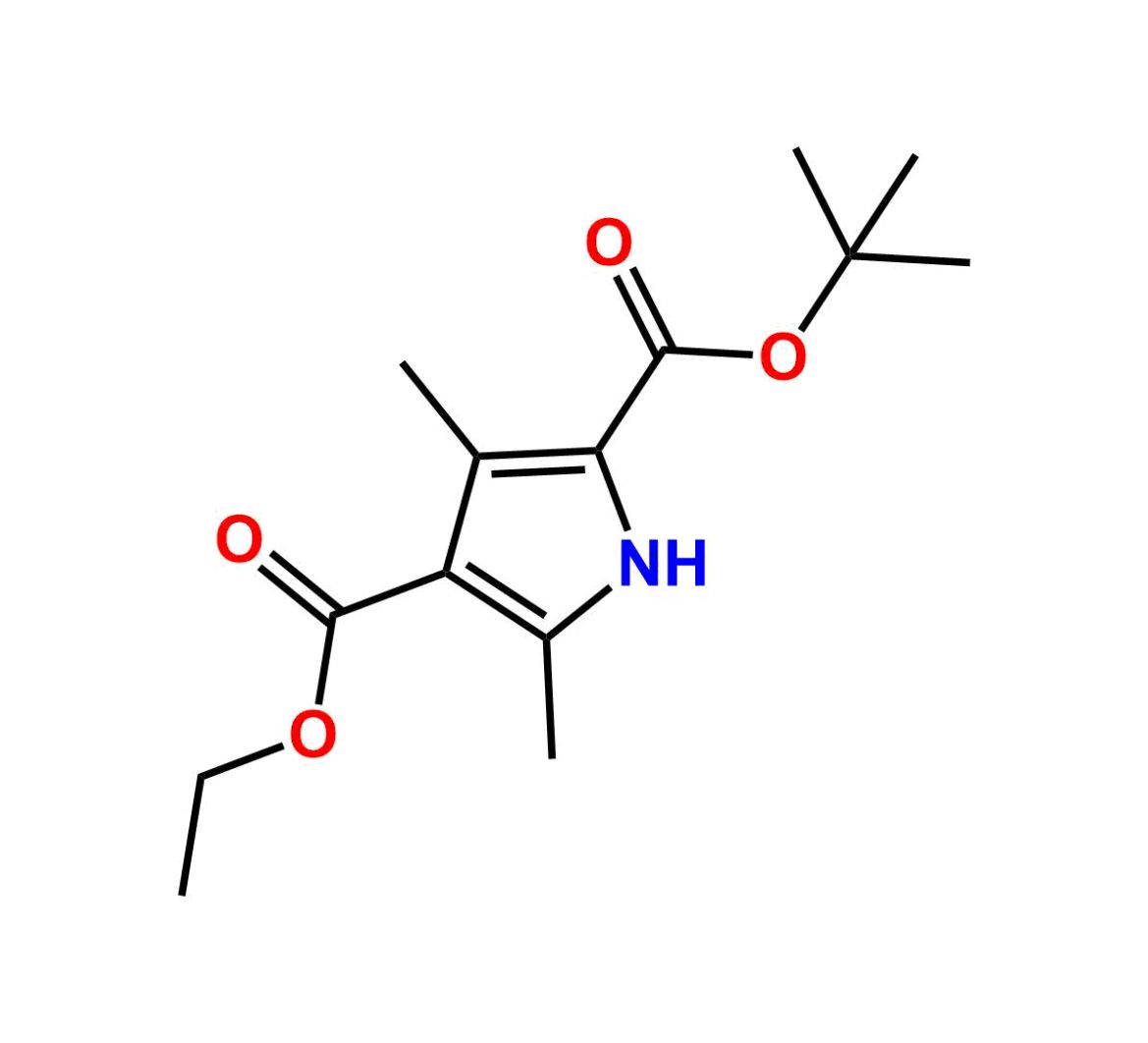
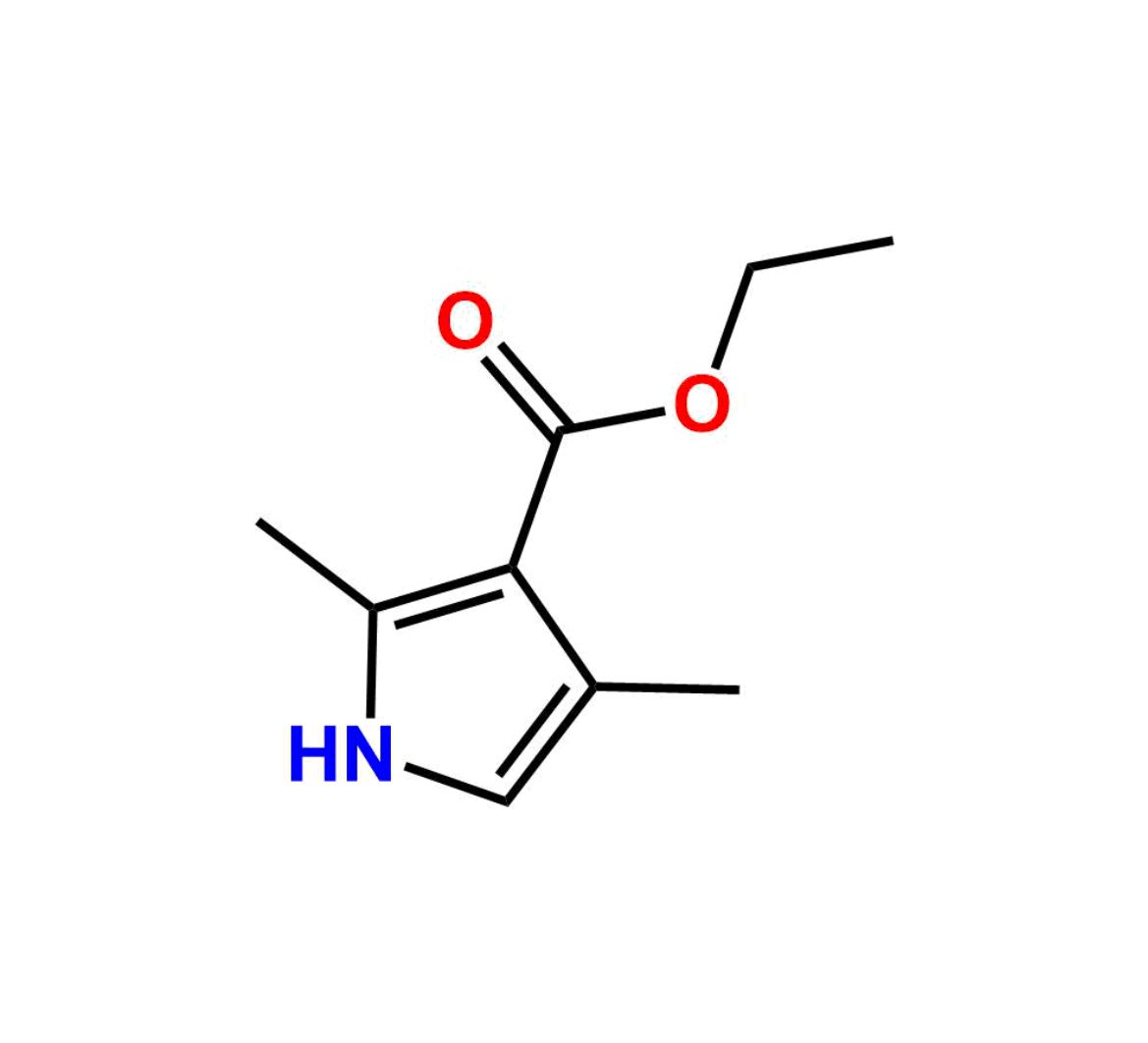
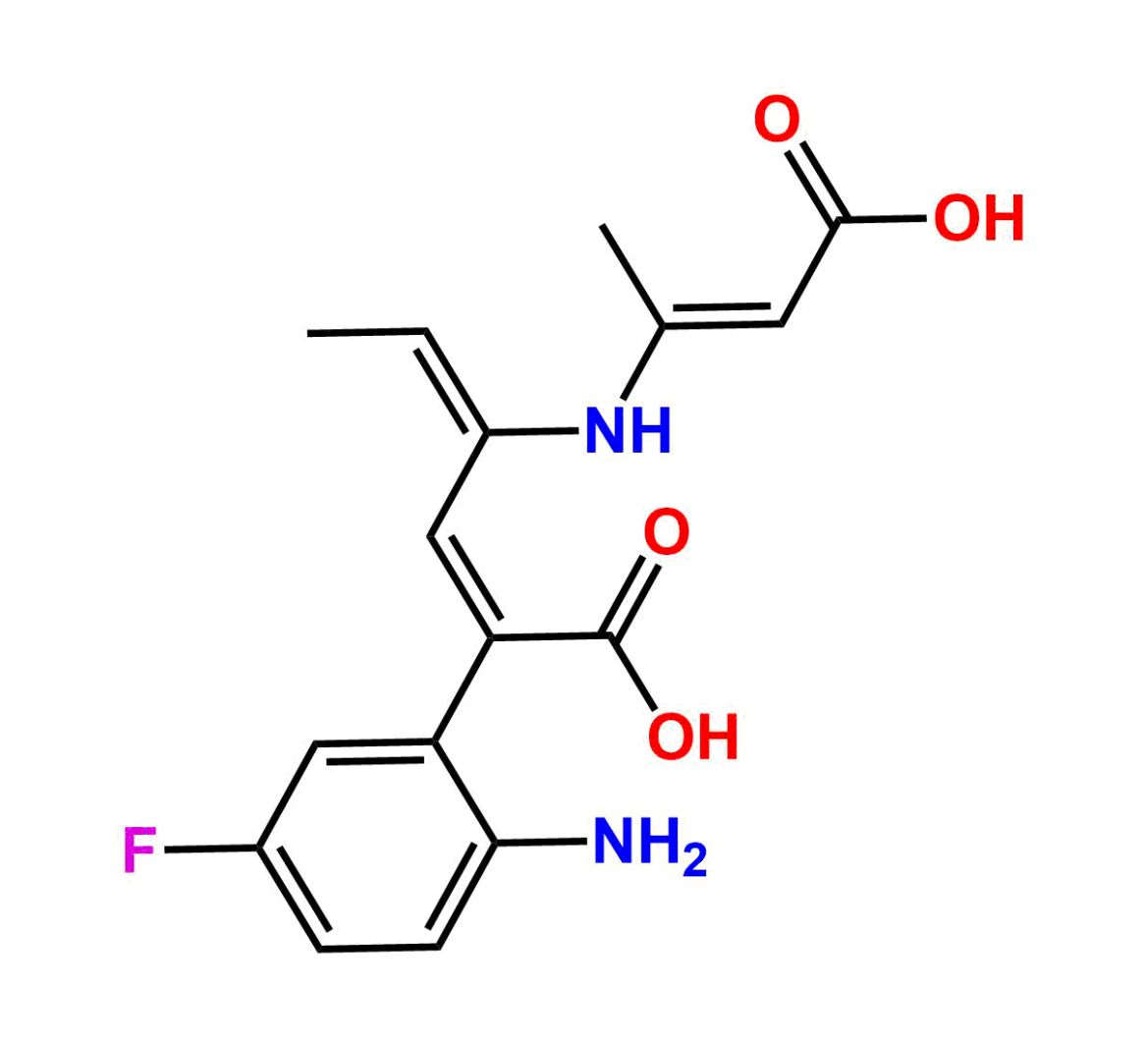
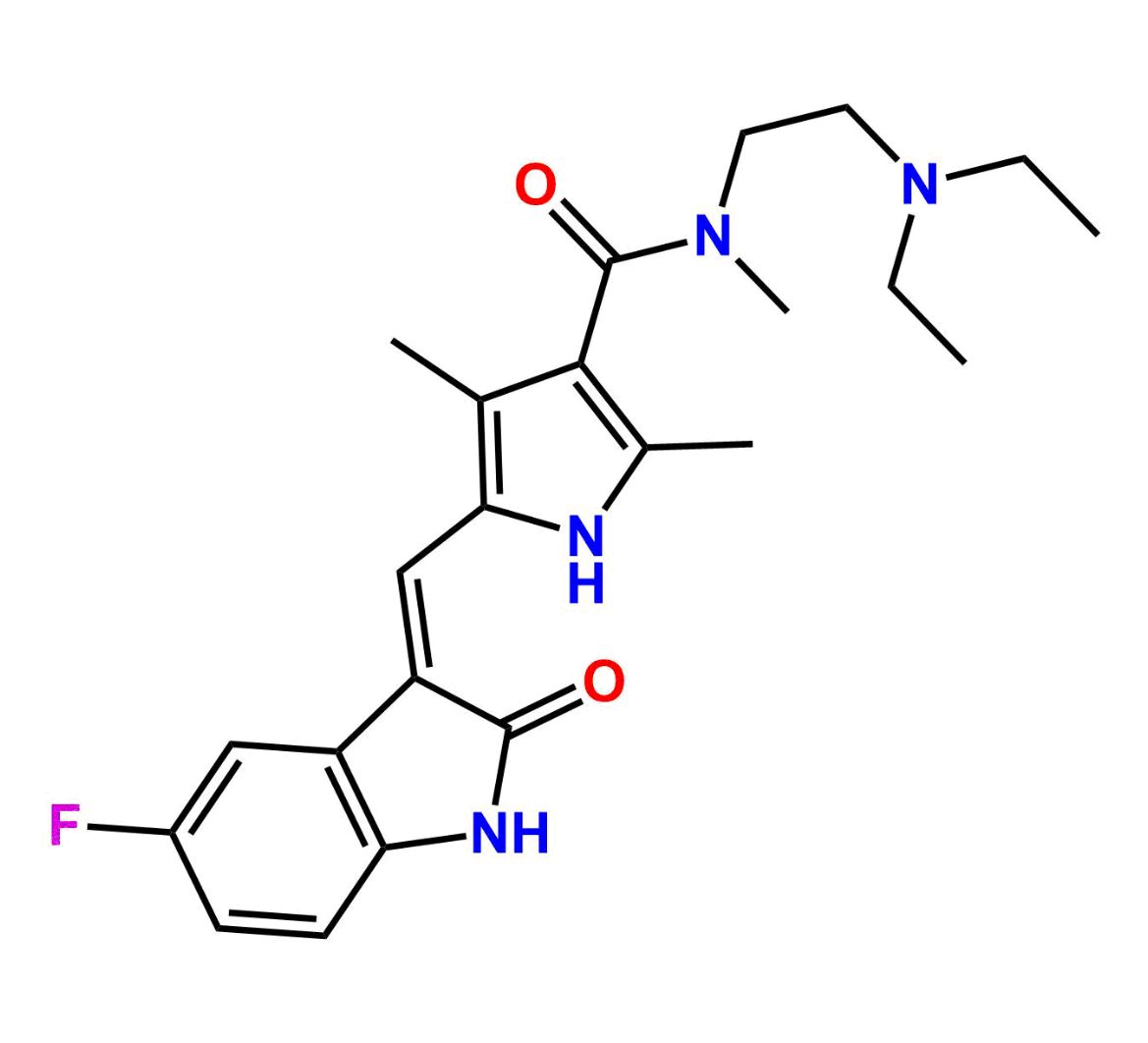
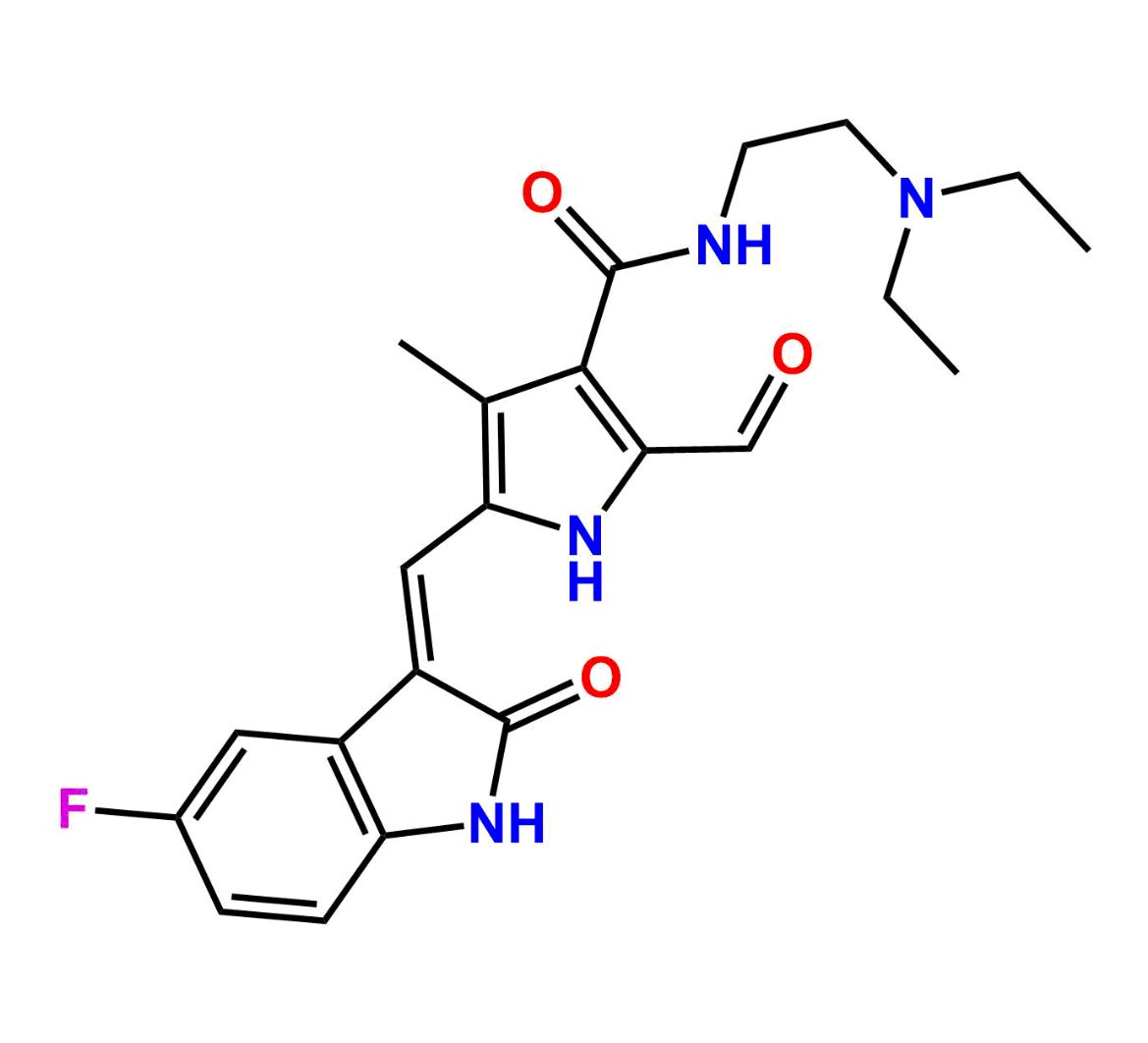
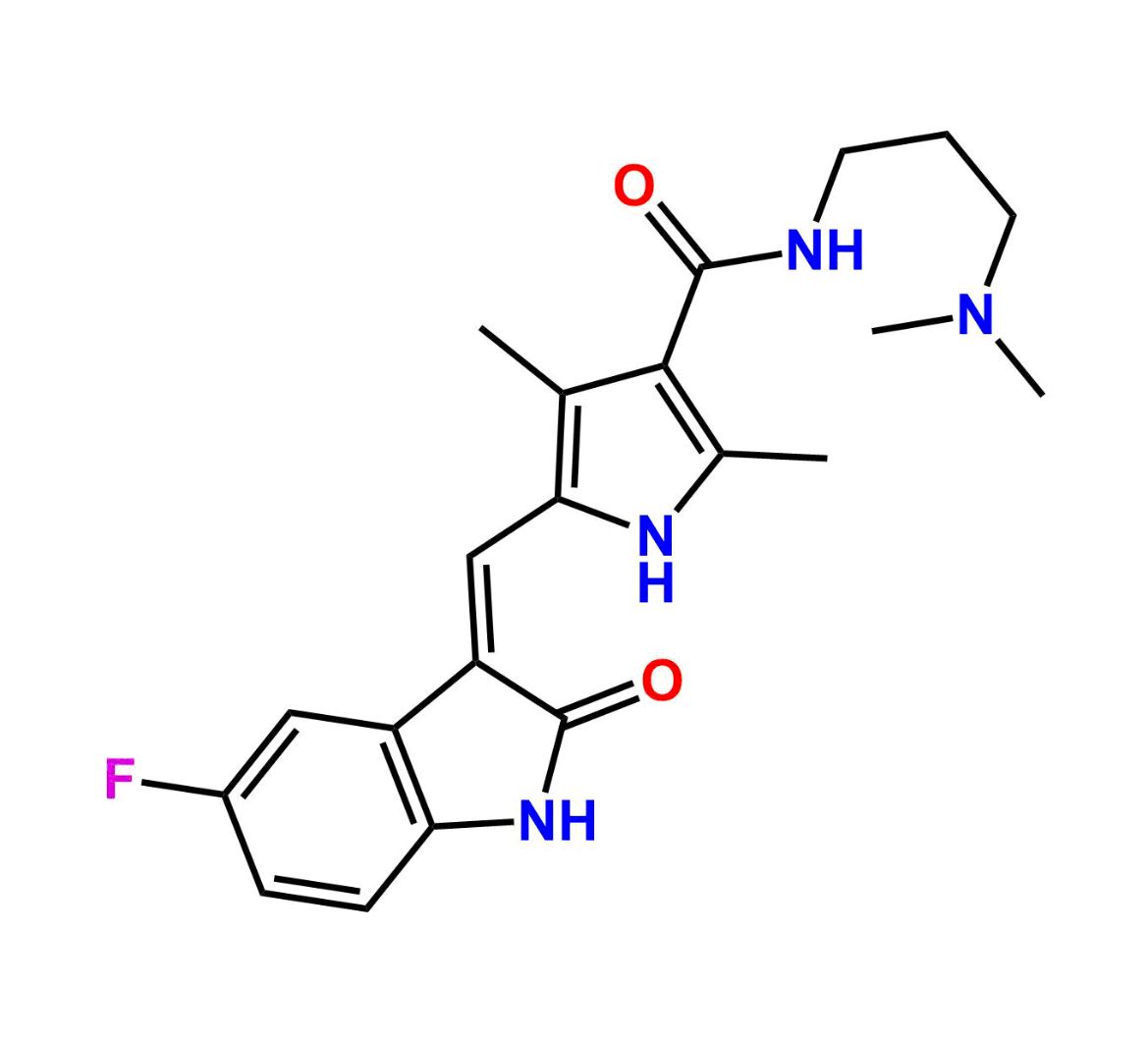
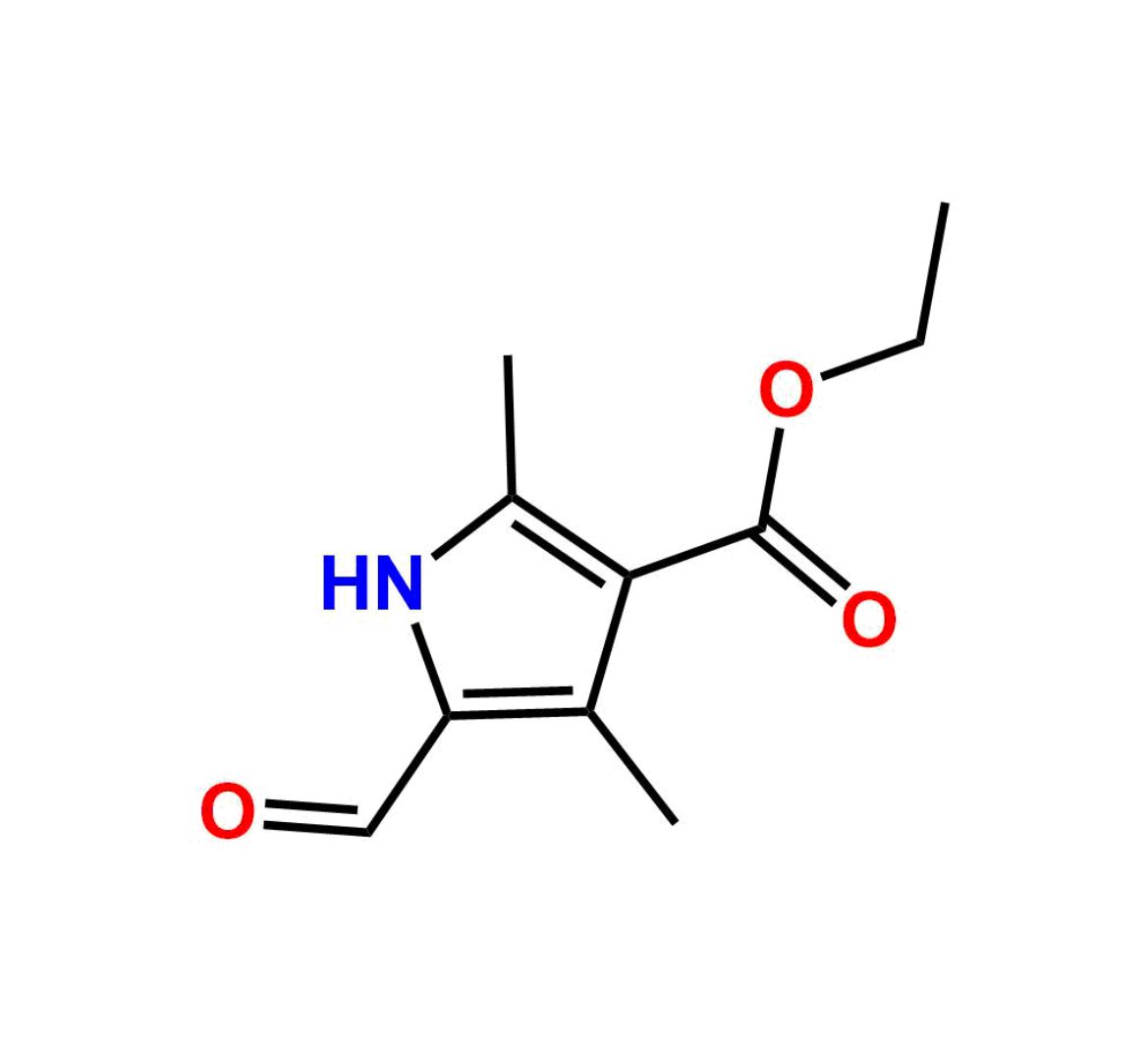
This impurity is related to Sunitinib Impurity 21 is provided with comprehensive characterization data in accordance with regulatory guidelines. It is suitable for use in analytical method development, method validation (AMV), Quality Control (QC) applications for Abbreviated New Drug Applications (ANDA) and commercial production of Asenapine.