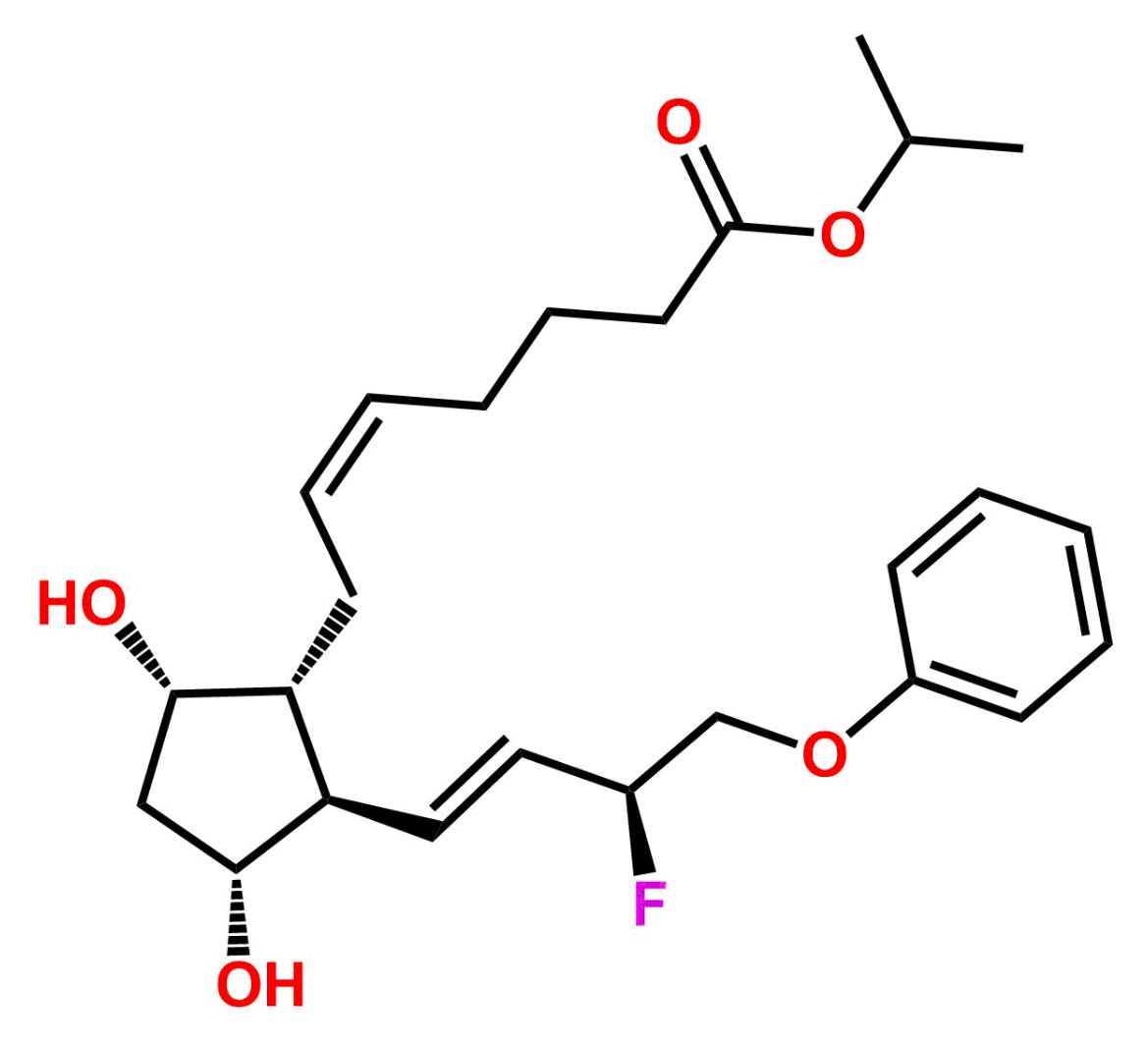
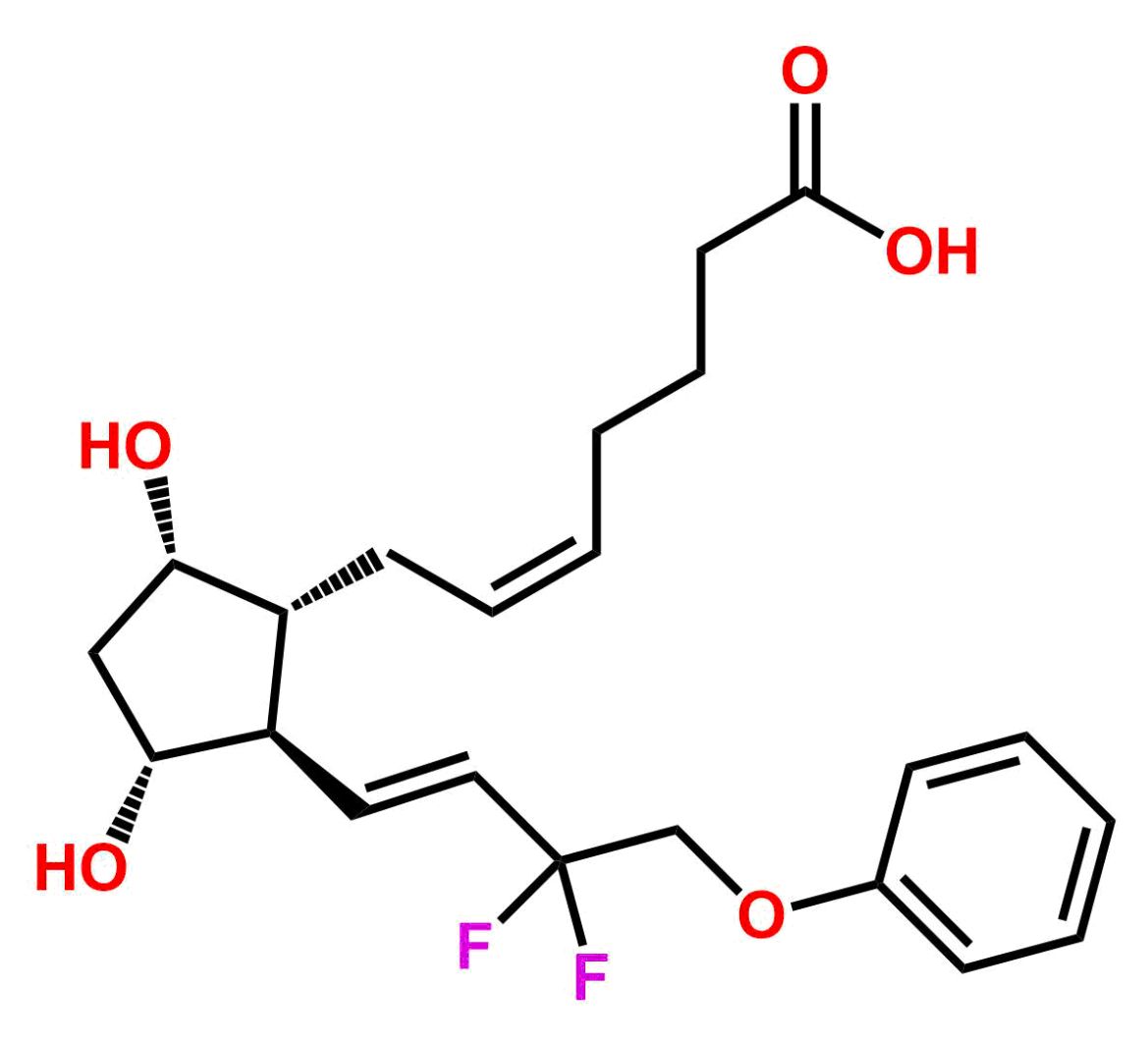
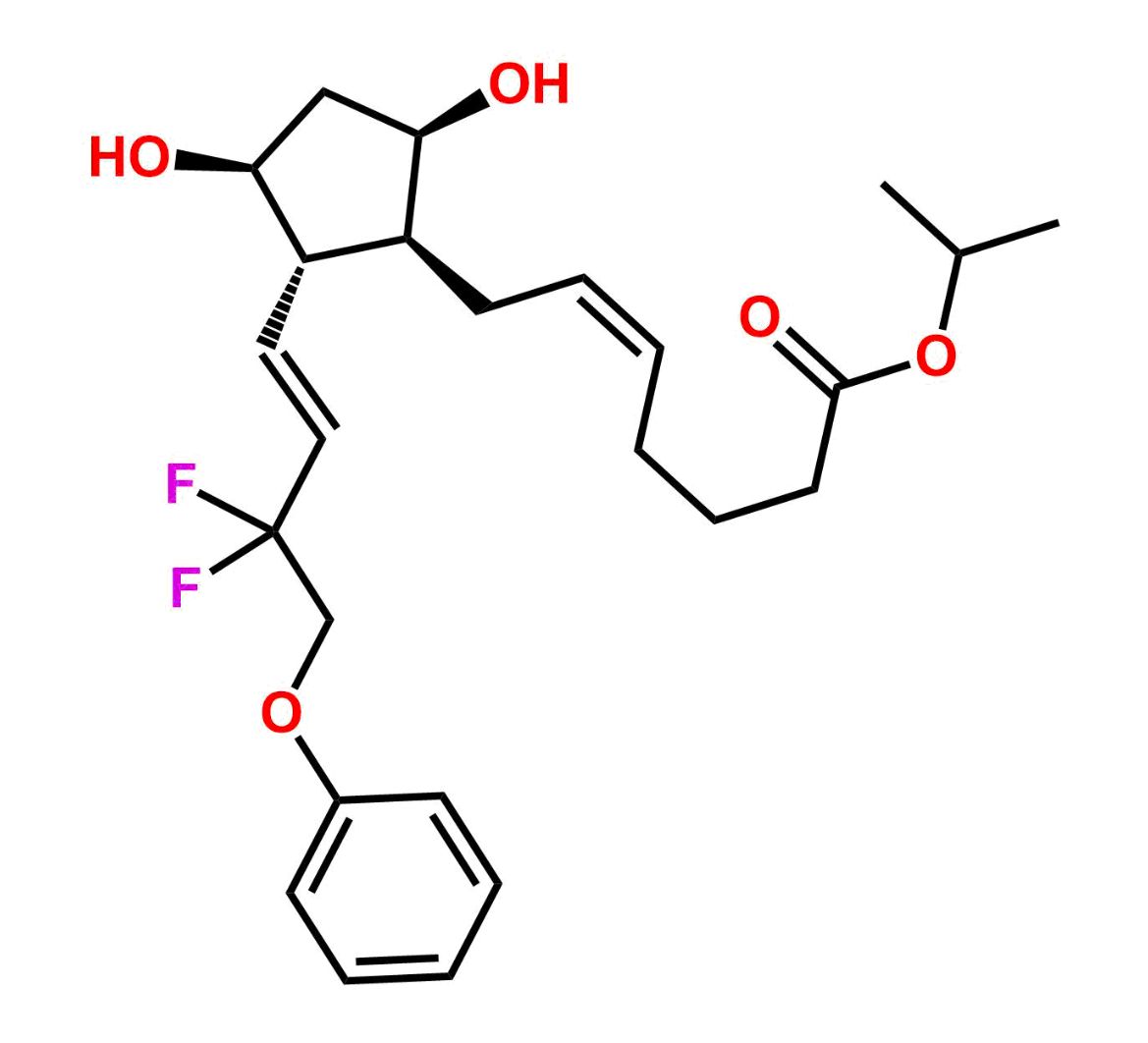
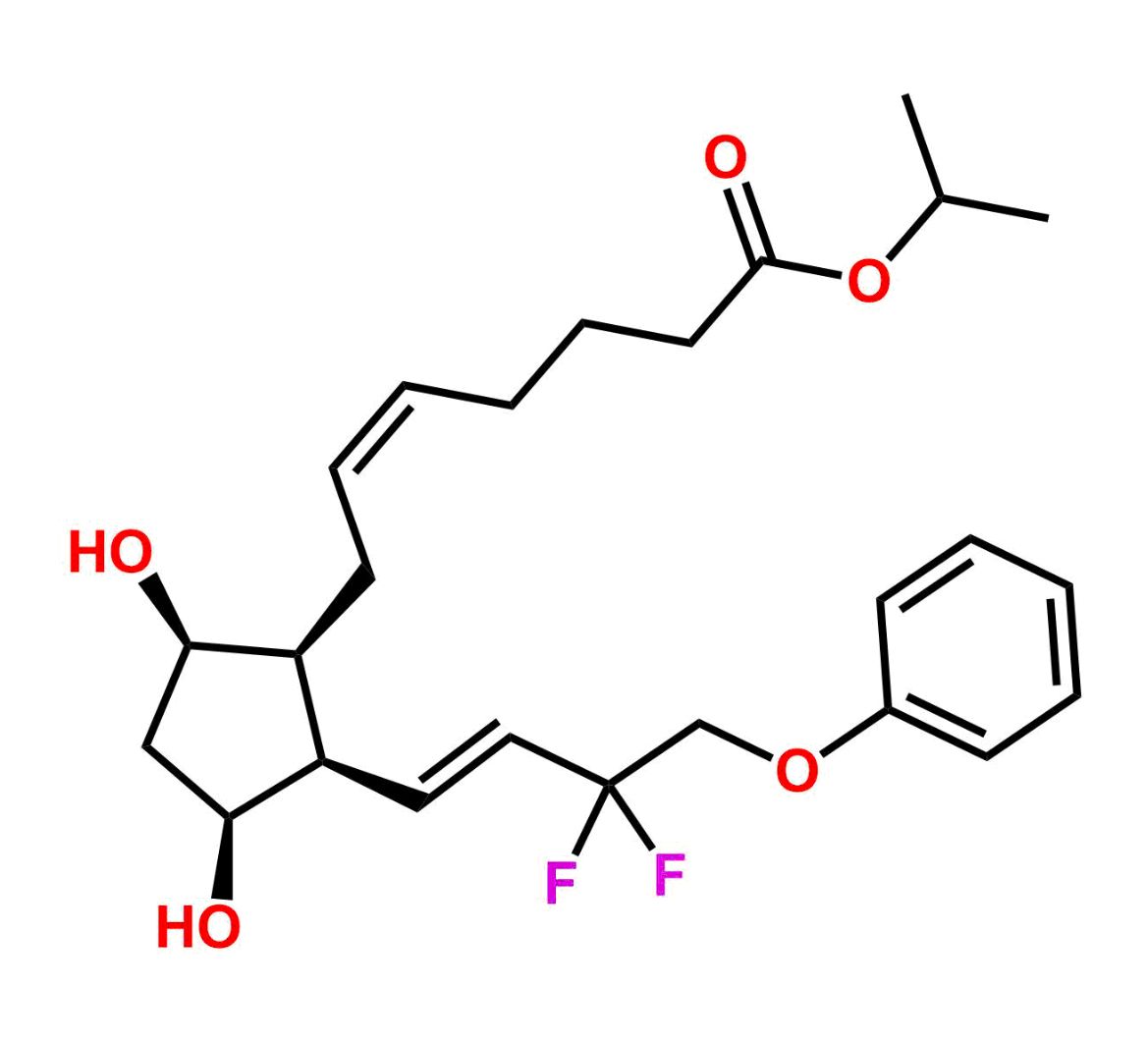
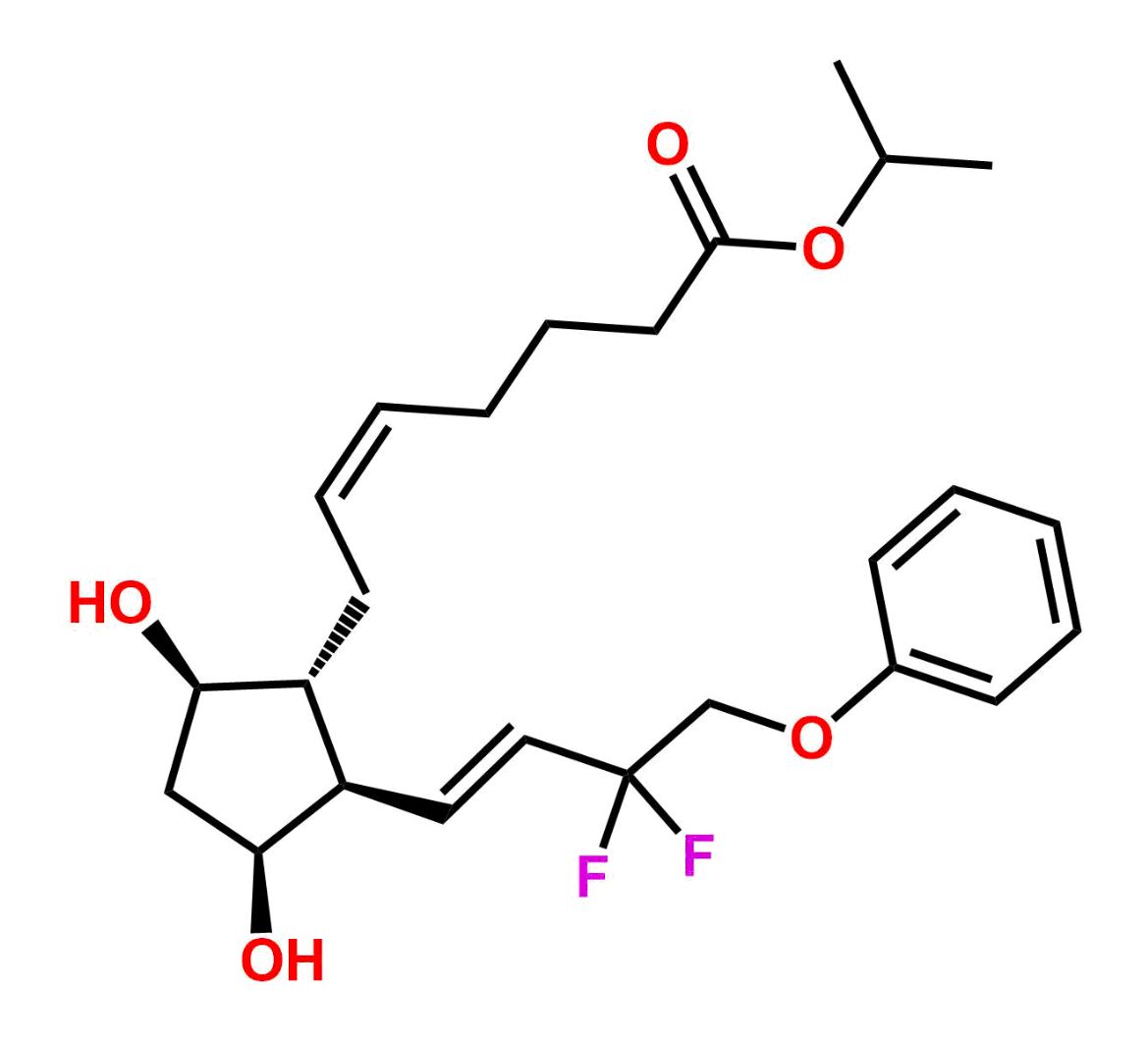
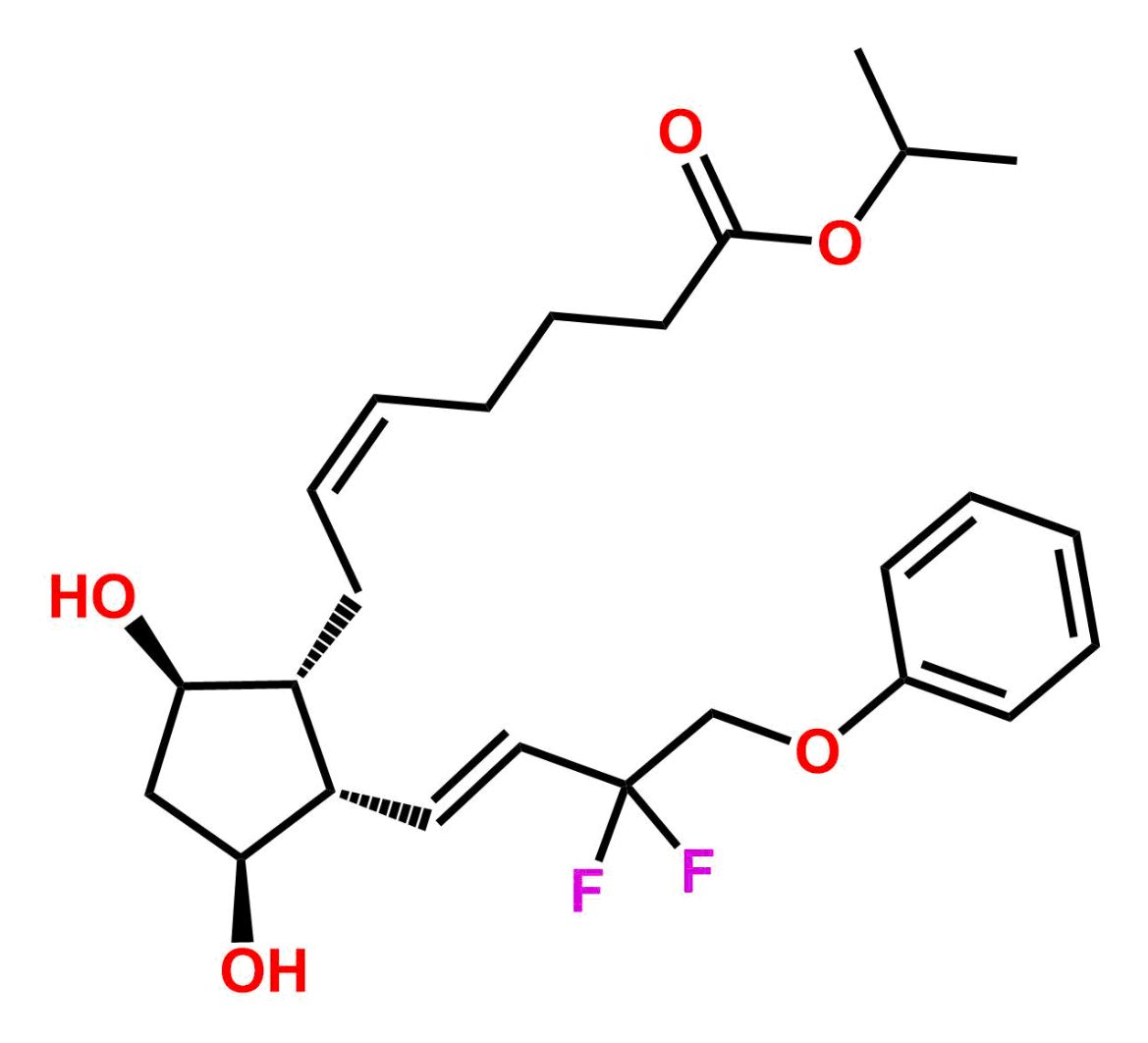
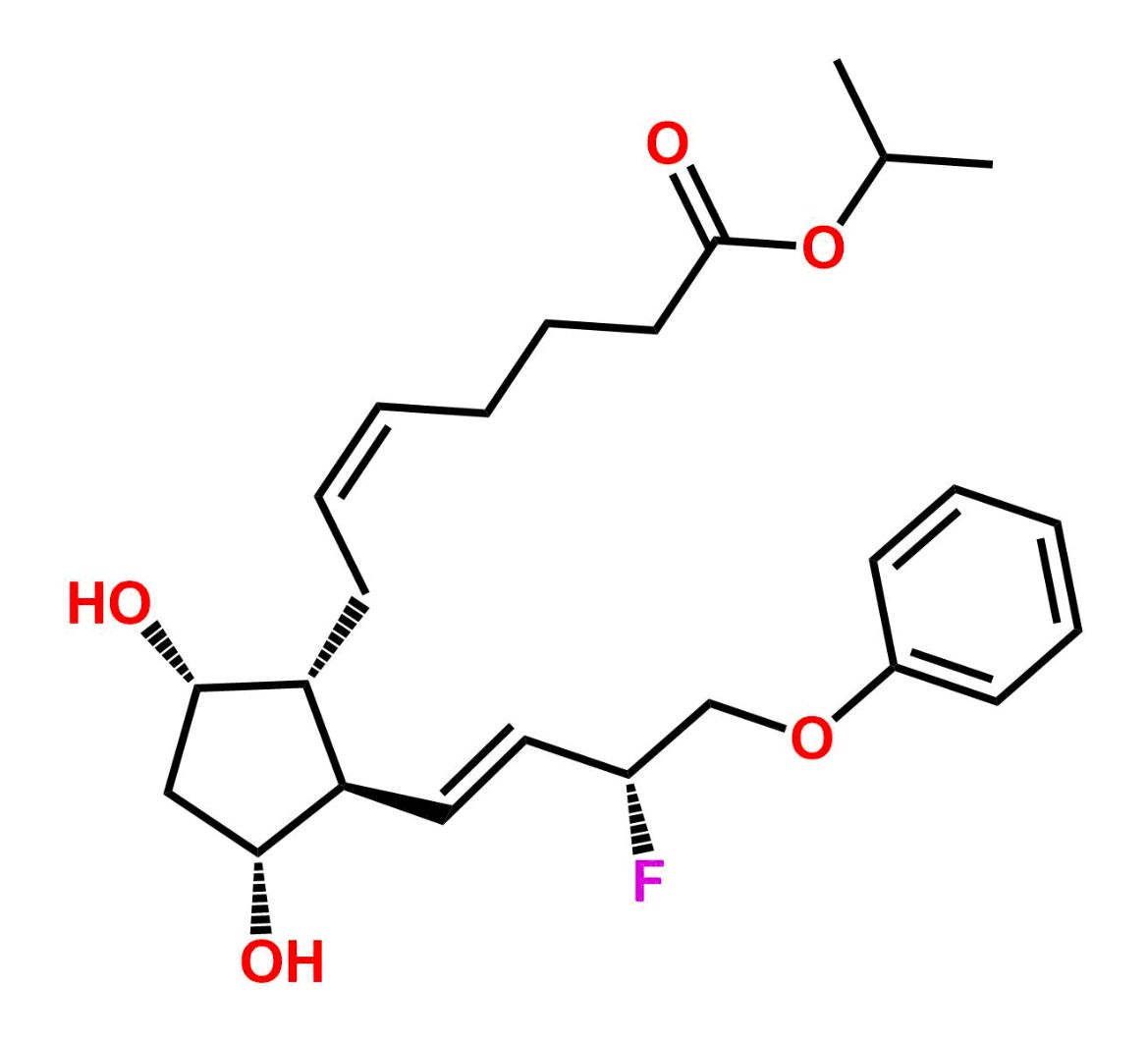
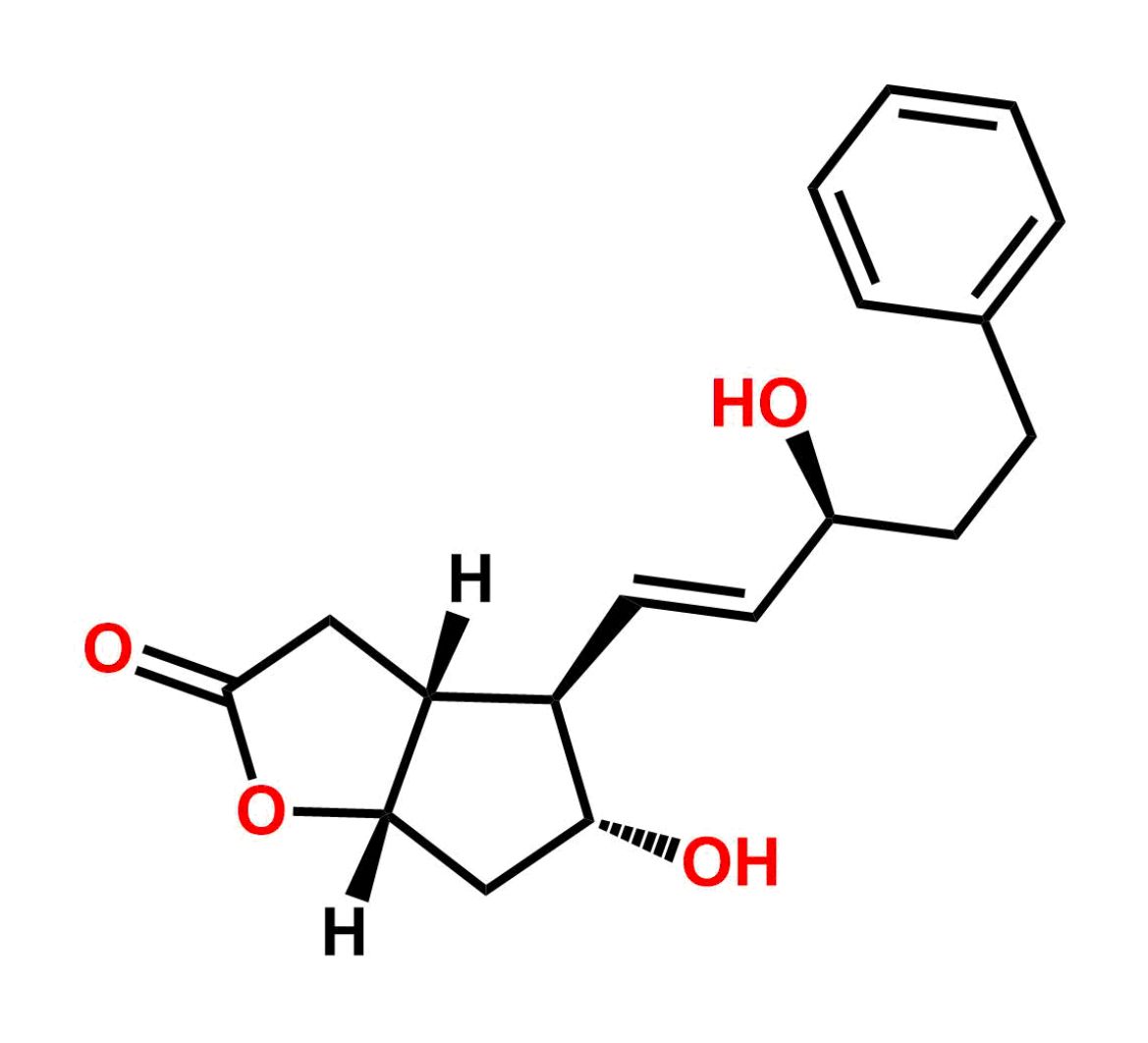
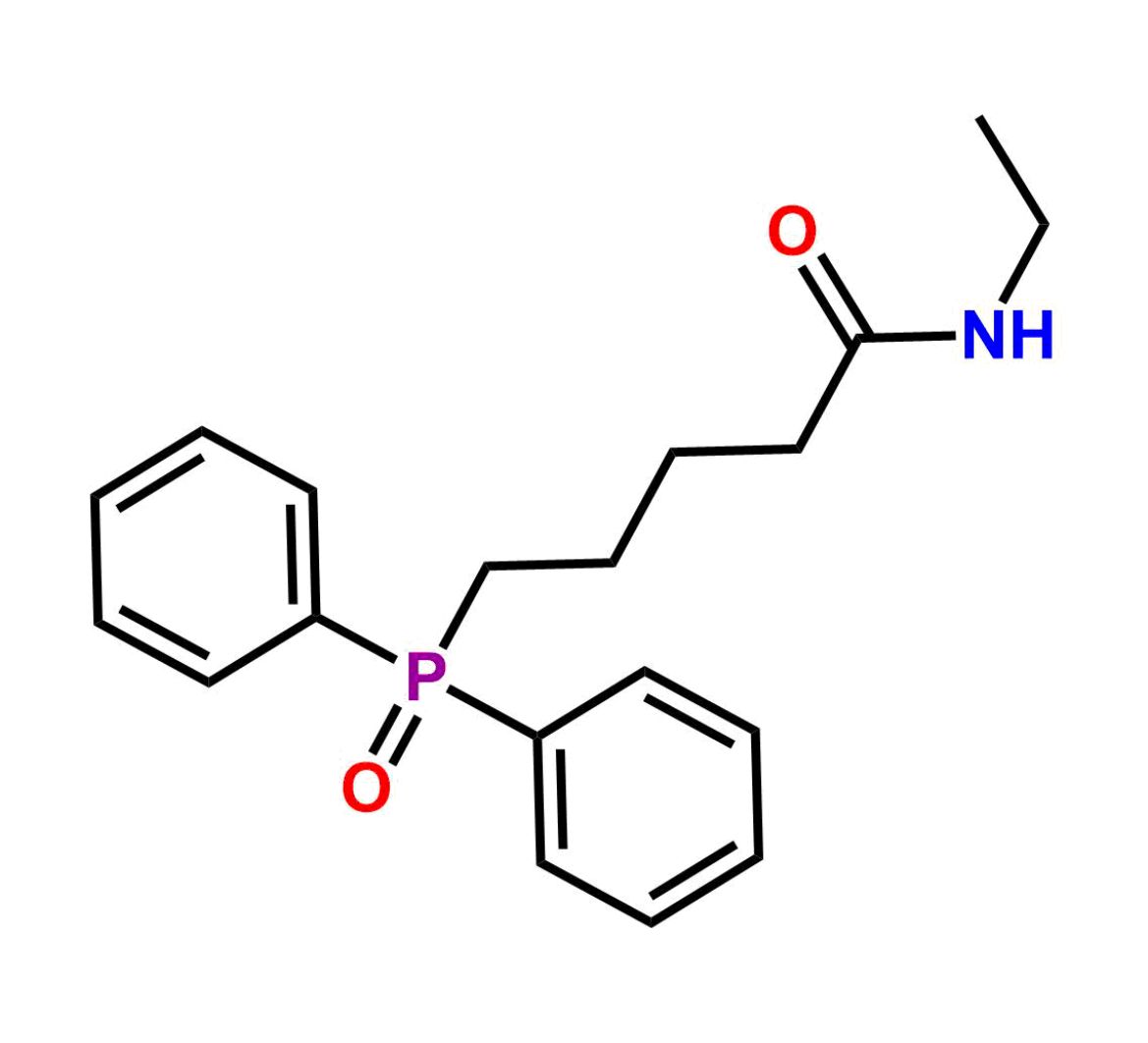
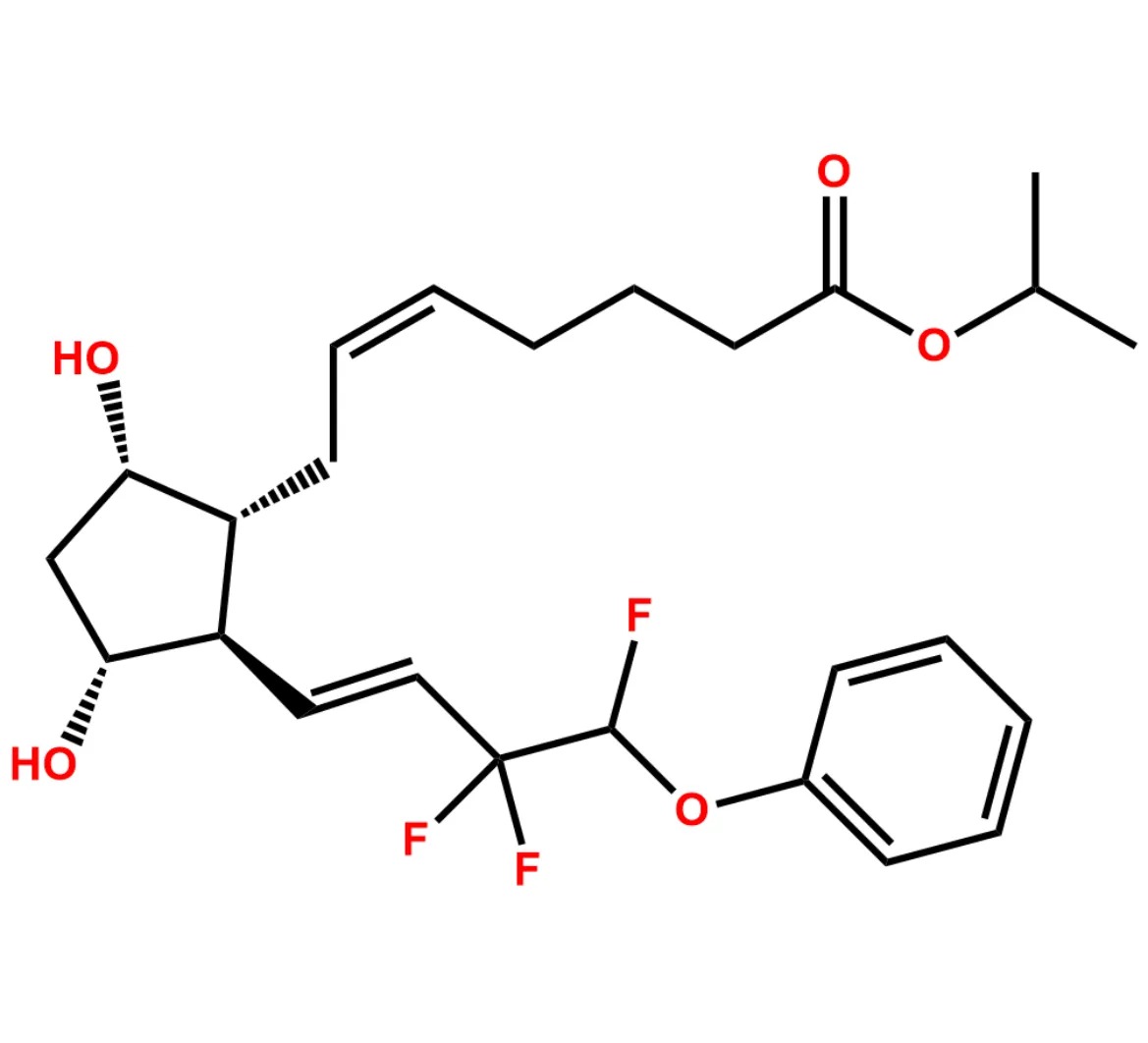
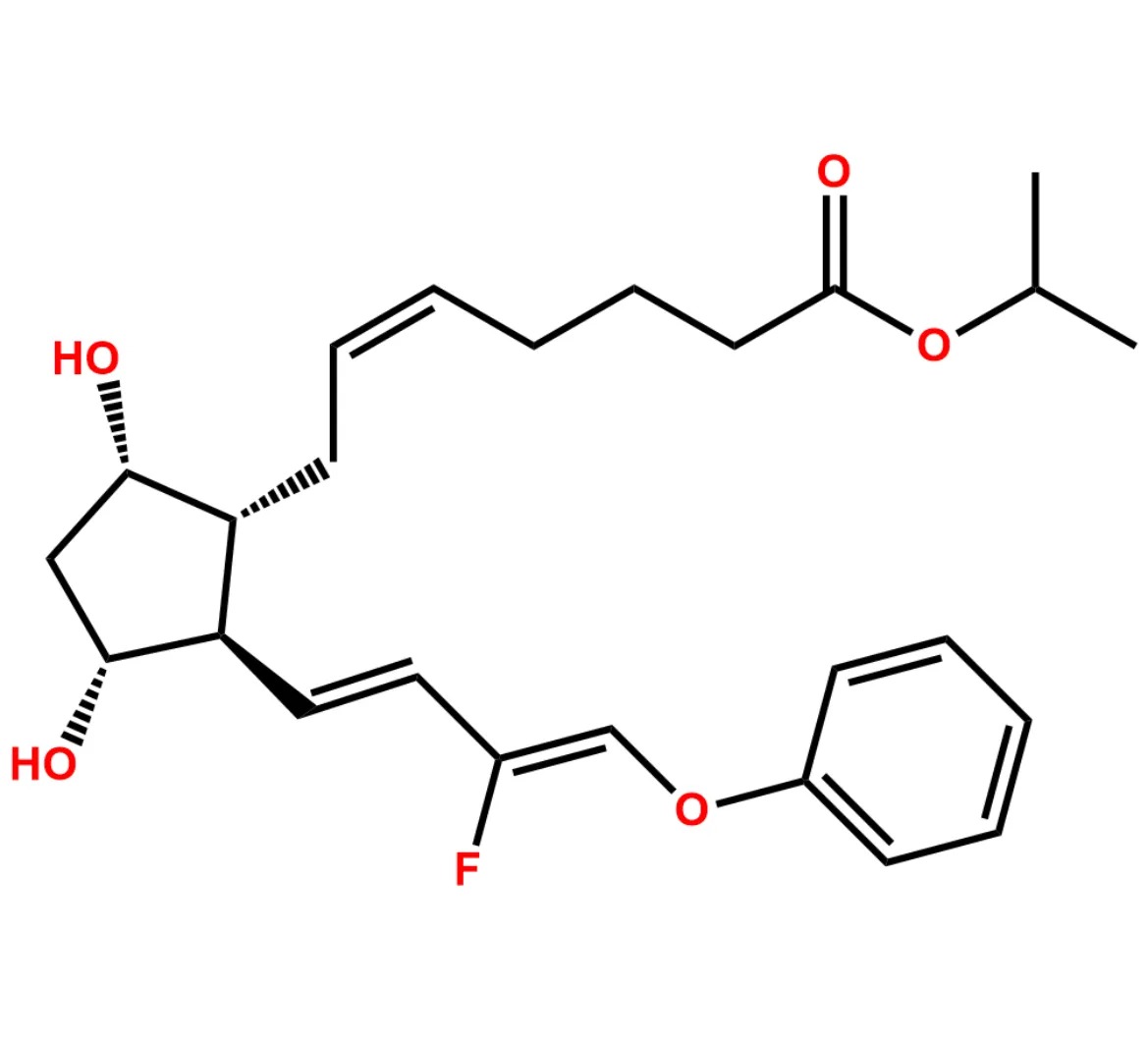
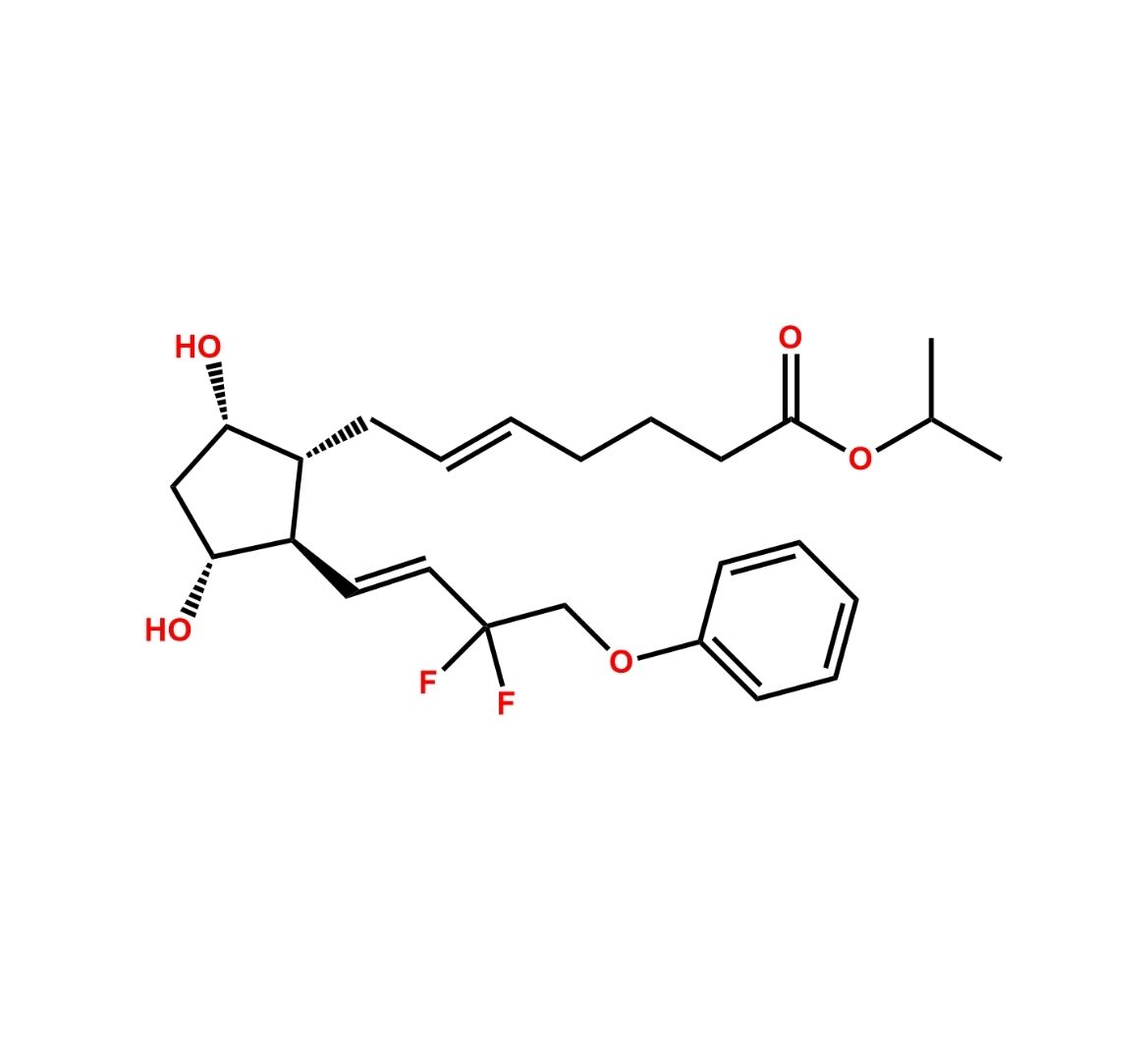
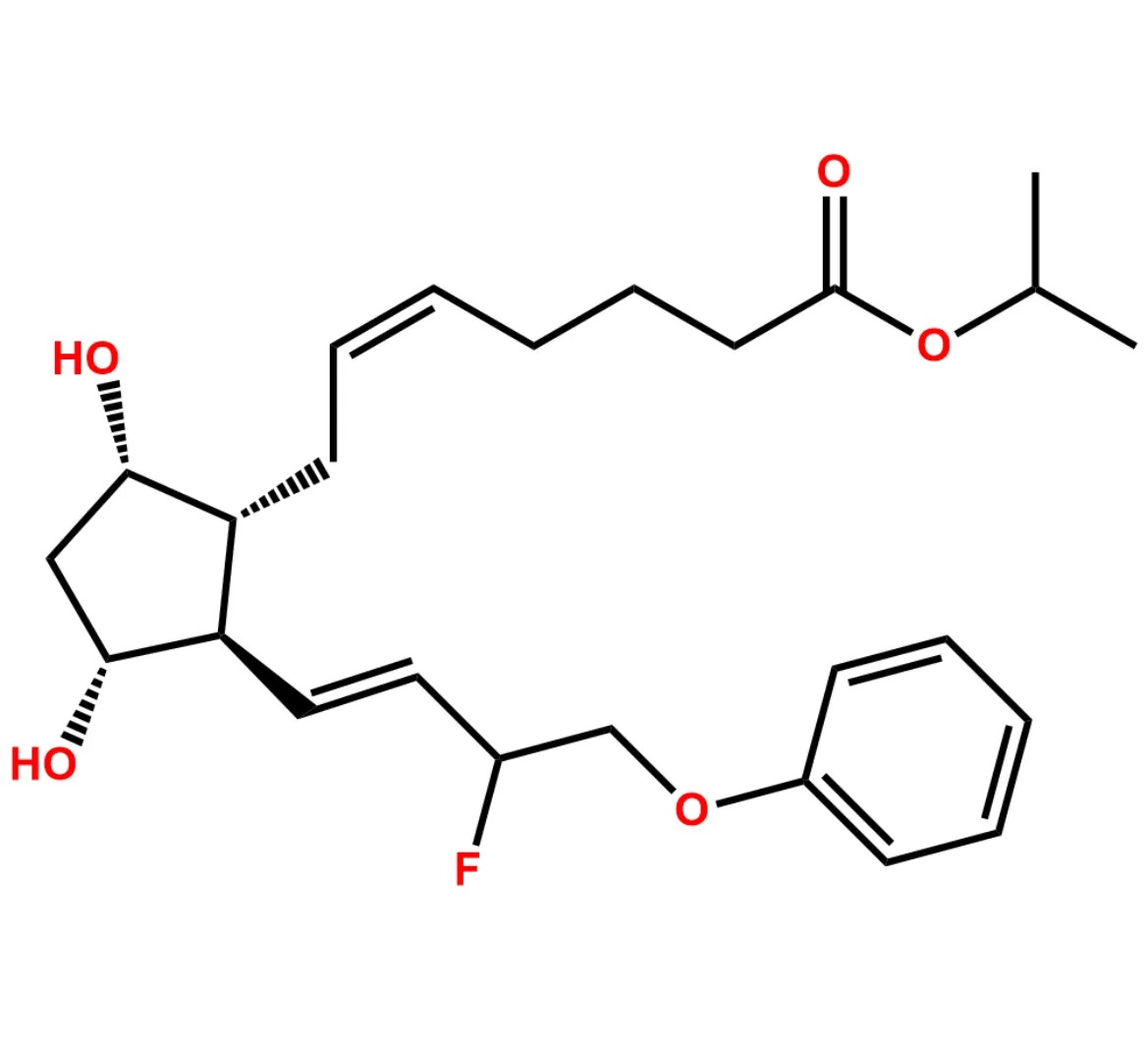
This impurity is related to Tafluprost Impurity 2 is provided with comprehensive characterization data in accordance with regulatory guidelines. It is suitable for use in analytical method development, method validation (AMV), Quality Control (QC) applications for Abbreviated New Drug Applications (ANDA) and commercial production of Asenapine.
Tafluprost Impurity 2 is used as a reference standard in analytical research. It ensures consistency of formulations.
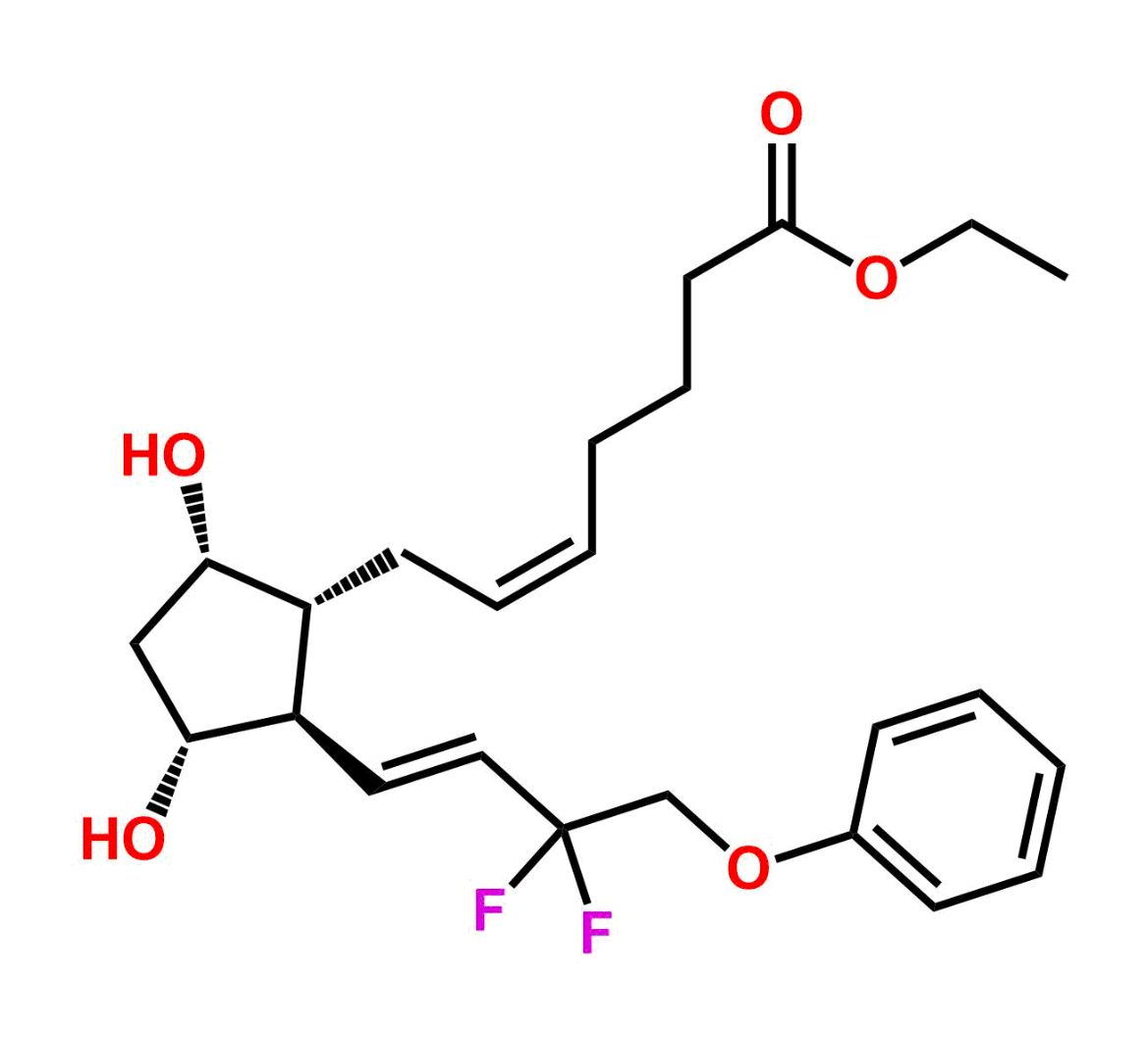
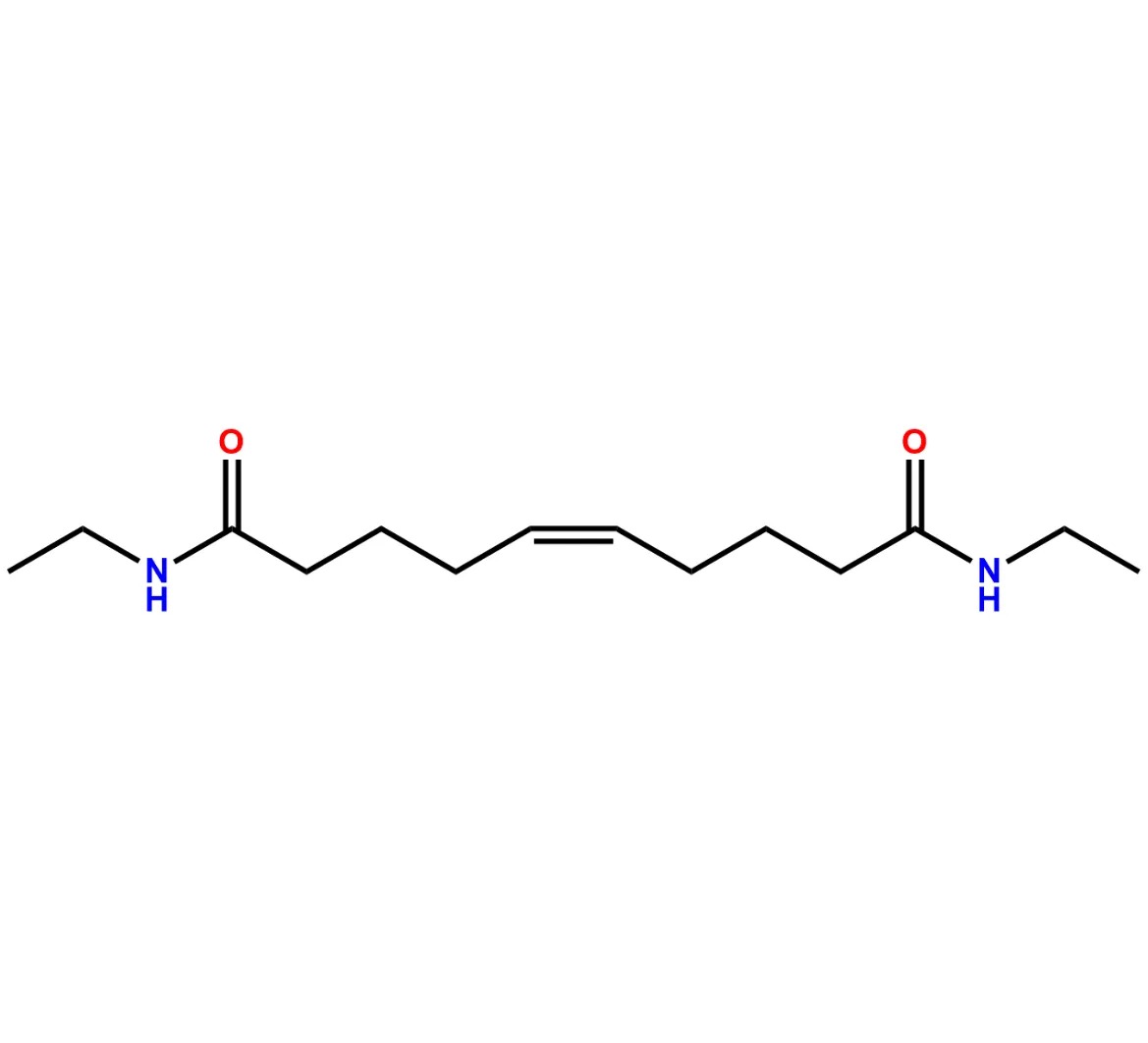
Chemical Name:(Z)-N1,N10-diethyldec-5-enediamideCountry of Origin: India Product Category: Impurity Reference StandardAPI NAME: Tafluprost Molecular Formula: C14H26N2O2
Molecular Weight: 254.4
Storage: Store in a cool, dry place.